Vascular occlusion following injection of volumi sing materials is not a novel phenomenon. It was first described over 100 years ago, when injecting petroleum jelly to correct facial deformities caused by disease processes or injury, was practi sed. Migration, infection and infl0ammatory responses were also reported, much as they are today with modern fillers, although clearly much less often latterly, as a result of aseptic technique and biocompatible materials. Adverse vascular events (AVEs) have also been reported with other injected therapeutic substances. Nicolau syndrome was first described as livedo dermatitis and gangrene in 1925, following treatment of syphilis with bismuth salts (Kim and Chae, 2015). Subsequent cases, sometimes described as embolia cutis medicamentosa, have also been reported with non-steroidal anti-inflammatory agents (NSAIDs), steroids, antibiotics, sclerosing substances, vaccines, alpha antagonists, sedatives, immunomodulators, recombinant interferon and tumour necrosis factor. In principle, injected substances which are able to occlude due to their viscosity or their eff ects on vessel linings may cause AVE.

Estimates of incidence vary widely, as a result of the total number of filler injections administered in any given time period being unknown, reluctance on behalf of injectors to report cases when they arise and because less severe AVEs may resolve without significant sequelae and remain undiagnosed. Schelke et al (2020) estimated an incidence of 1:5 300–1:8 000 (0.01–0.02%); however in 2014 Beleznay’s group had more than double the number in their observational study of 14 355 consecutive injections resulting in 8 cases (0.05%) of AVE.
Some three pathophysiological mechanisms of vascular compromise have been cited: direct emboli sation due to intravascular injection ; compression of vessels from adjacent filler material, possibly compounded by swelling related to bruising and/or the hydrophilic properties hyaluronic acid (HA); and spasm of vessels (King et al 2020). The latter has been difficult to demonstrate experimentally but this phenomenon which protects adjacent tissues from spread of potentially toxic substances may be the reason why despite the richly anastomotic vascular network of the face, significant ischaemic damage can ensue following occlusion of a single vessel.
In order to mitigate risk as much as possible, careful assessment for each treatment should be undertaken. A detailed history with specific enquiry regarding previous facial surgery or trauma should be made. Scarring tethers tissues, including blood vessels, rendering them less able to slide away from an advancing cannula. Previous rhinoplasty surgery may leave nasal vasculature compromised and unable to withstand any further vascular insult. Clients may not always divulge this information, even on direct questioning. Some may not remember a small scar resulting from a childhood injury; occasionally, this information may be consciously withheld, if the desire for a particular treatment has been declined elsewhere on these grounds. Close examination of the face looking for scars is therefore an important part of the assessment. Clear documentation of the history and clinical findings will be invaluable in the event of later litigation, so is worth the investment in time.
Risk according to incidence of injection site for AVE in general may, in part, be reflected by the popularity of the area of treatment. The FDA medical device reports from 2025–2020 reported the highest incidence of all AVE from perioral injections (38%), nasolabial (18%) and nose 10%; the highest risk areas for AVE resulting in blindness were close to the midline and in the superior two-thirds of the face; glabella (39%), nose (26%), nasolabial fold (13%) and forehead (12%), according to Belaznay’s 2015 review of the world literature. In terms of depth, risks appear generally to be greatest within the soft tissue between bone and dermis, where vessels may run a tortuous and unpredictable course and can vary both between individuals and on opposite sides of the same individual.
The type of filler injected also influences risk stratification. Fat injections have been historically associated with higher risk; perhaps because it is often injected in the middle lamellar soft tissues in multiple, rapid passes. HA fillers have become the most prevalent material associated with AVE in recent years, probably because of the rapidly increasing numbers of injected HA fillers performed over that time. Monophasic HAs with high G-prime appear to have the greatest propensity to occlude vessels. A further histopathological finding is that HAs have a unique ability to incite a platelet response within the vessel so that the mechanical effects are further compounded by an occlusive biological response (Soares, 2022). Polylactic acid (PLLA) and hydroxyapatitebased fillers appear to carry lesser risk, perhaps because of their less viscous carrier fluid, however, the ability to easily reverse AVEs with hyaluronidase may lead to worse outcomes in those cases which do occur.
The introduction of injection via cannula was hoped to have eliminated the risk of vascular occlusion, however this has not been the case; cannulae have been found both in laboratory testing and clinical practice to penetrate vessel walls, especially those finer than 25 gauge and in areas of scarring or type 2 SMAS, such as the perioral area. Identification of vessels pre-injection using ultrasound can be useful in avoiding them; however, for many injectors the cumulative expense of the time added to each procedure, the training required to achieve competence and the technology itself may be prohibitive.
Whether to aspirate or not remains a point of lively discussion between injectors; indeed, while a positive flashback is a reliable sign of vascular cannulation, a negative does not exclude it. The apparently variable reliability comes down to the Hagen-Poiseuille Equation, the law of non-compressible fluid mechanics, where flow of the fluid through a cylindrical tube of fixed diameter is determined by the variables of length and diameter of the tube, viscosity of the fluid and pressure. This determines that it is very unlikely to get a positive aspirate via a long, fine=bore cannula, but much more likely through a short, larger gauge needle.
Whatever method of delivery is used, slow injection is recommended, as a smaller bolus, if injected intravascularly, will cause less distal ischaemia than a larger one. It also allows for close observation of the tissues during injections so that the earliest signs of AVE are immediately detected.
Five stages of ischaemic change have been described
- Stage 1 Blanching, occurring within seconds of the AVE, representing cessation of flow within the angiosome supplied by the occluded vessel.
- Stage 2 Livedo reticulares, a net-like bluish discolouration of the skin in the same distribution. This appears after minutes and lasts up to 36 hours.
- Stage 3 Pustular. Beyond 36 hours, the dysfunction of ischaemic sweat glands and change in pH of the skin allow overgrowth and invasion of skin commensals such as Staphylococcus aureus.
- Stage 4 Coagulative. Within 72 hours, the skin begins to undergo haemorrhagic necrosis and cell lysis. Erythema develops around the periphery of the angiosome.
- Stage 5 Devitalised tissue. After 72 hours, the affected area may be ‘wet’, ie moist sloughy and yellow/green or dark, dry and hard.
Intervention at the beginning of this process can prevent this cascade of damage. It is therefore important to have your team trained and hyaluronidase immediately to hand.
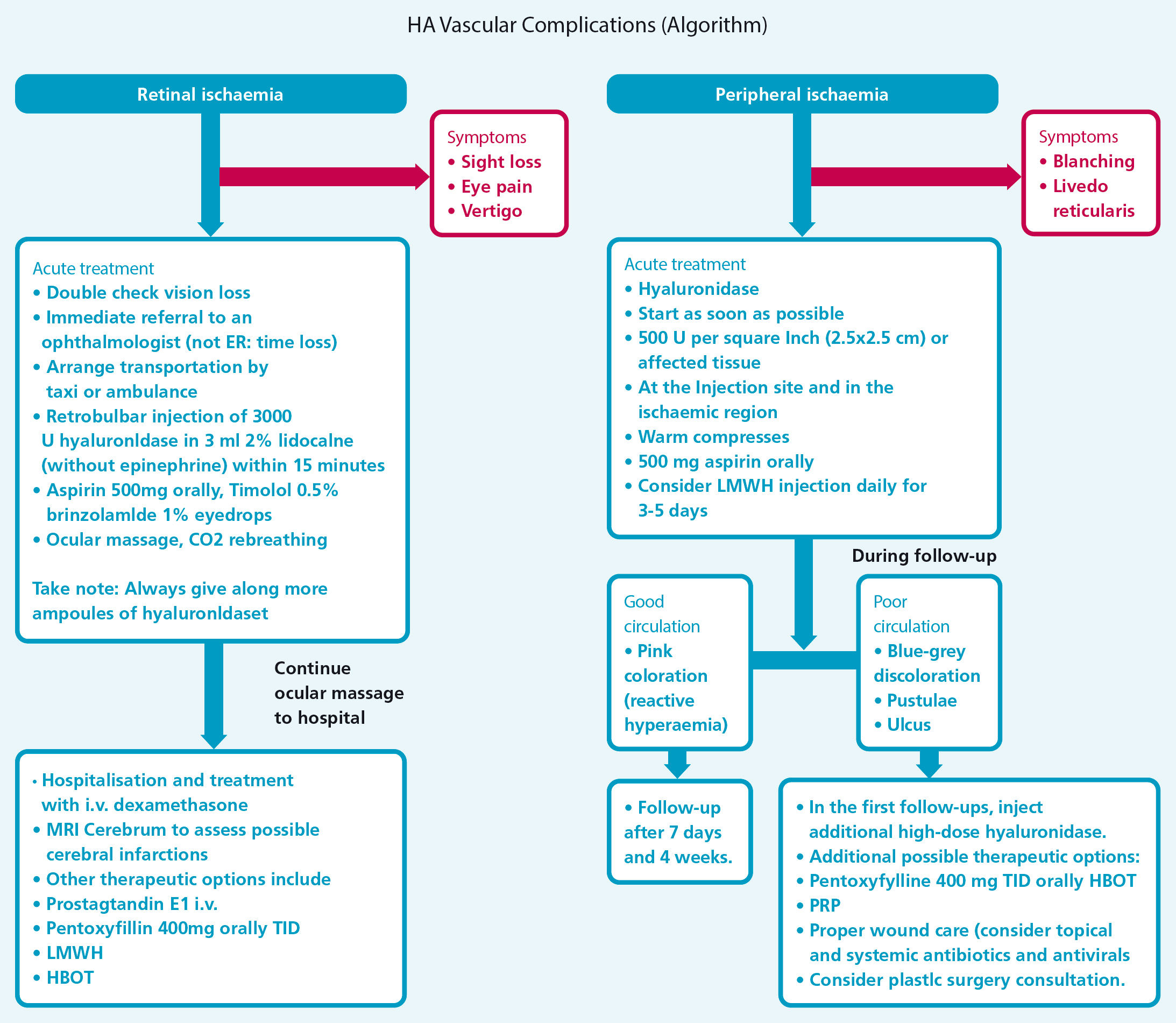
Ophthalmic artery occlusion (OAO) and its potentially life-changing effects mandate specific attention. In addition to the pain experienced in stage 1, nausea, double vision, visual loss, upper eyelid ptosis and pupillary dilatation may also feature, depending on exactly where the embolus is sited. Visualisation of the retina would reveal the so-called ‘cherry red spot’ appearance seen as a result of oedematous, ischaemic inner retinal layers contrasting with the central foveal area where they are absent. The optic nerve head may be swollen or pale. The prognosis for restoration of sight in reported cases has been almost universally poor. Historical teaching on non-iatrogenic retinal artery occlusion surmised that recovery of function could be achieved if treatment was commenced within an hour of the event. More recently, research by Tobalem et al (2018) has suggested that the retinal ganglion cells in the inner retina are damaged irreparably after only 12-15 minutes. It is not possible to deduce from the literature how long after AVE treatment was commenced, but it might explain the grim prognosis for those who receive it after 15 minutes. Rarer but similarly devastating is cerebral embolism resulting in stroke (CVA). A worldwide literature review by Wang et al, published in 2022, reported 16 cases between 2016 and 2020. A small number, but a steady increase over consecutive 5-year periods, likely to reflect the ever increasing popularity of filler injections.Emergency measures for vascular occlusion can be found in Figure 1.
Conclusion
Once emergency treatment has been commenced in the clinic, it is important to refer the patient into an appropriate specialist ophthalmologist, stroke physician etc for ongoing care via emergency ambulance. This may involve intravenous infusion of acetazolamide (for OAO) and/or steroid, thrombolysis (for CVA), antibiotics, surgical debridement and reconstruction. Knowledge of local secondary care services so that you are able to liaise personally with these specialists is important, especially as many specialists may not yet have had to manage filler related complications and may appreciate guidance.
Ensuring your team regularly rehearses the protocol to follow in the event of an AVE in the same way as we practise regular basic life support or fire drills is highly recommended, so that if it should happen the sense of panic and chaos which might otherwise descend is avoided and the client is reassured that they are in safe hands, receiving appropriate care. It is also important that clear documentation is kept, despite the stressful circumstances. Allocating a team member to be note keeper and record the events as they happen will ensure an accurate record exists, which may prove invaluable later.
While the risk of AVEs cannot be entirely eliminated, implementing the above guidance will ensure the safest possible practice for your clients and reduced anxiety levels for you and your team if the unthinkable should occur even despite your best efforts.
Key points
- ■ Adverse vascular events can cause potentially life-changing consequences
- ■ Even in the most experienced hands and injectors must acknowledge that the risk is impossible to eliminate completely
- ■ Once emergency treatment has been commenced in the clinic, it is important to refer the patient into an appropriate specialist ophthalmologist, stroke physician etc for ongoing care via emergency ambulance



