
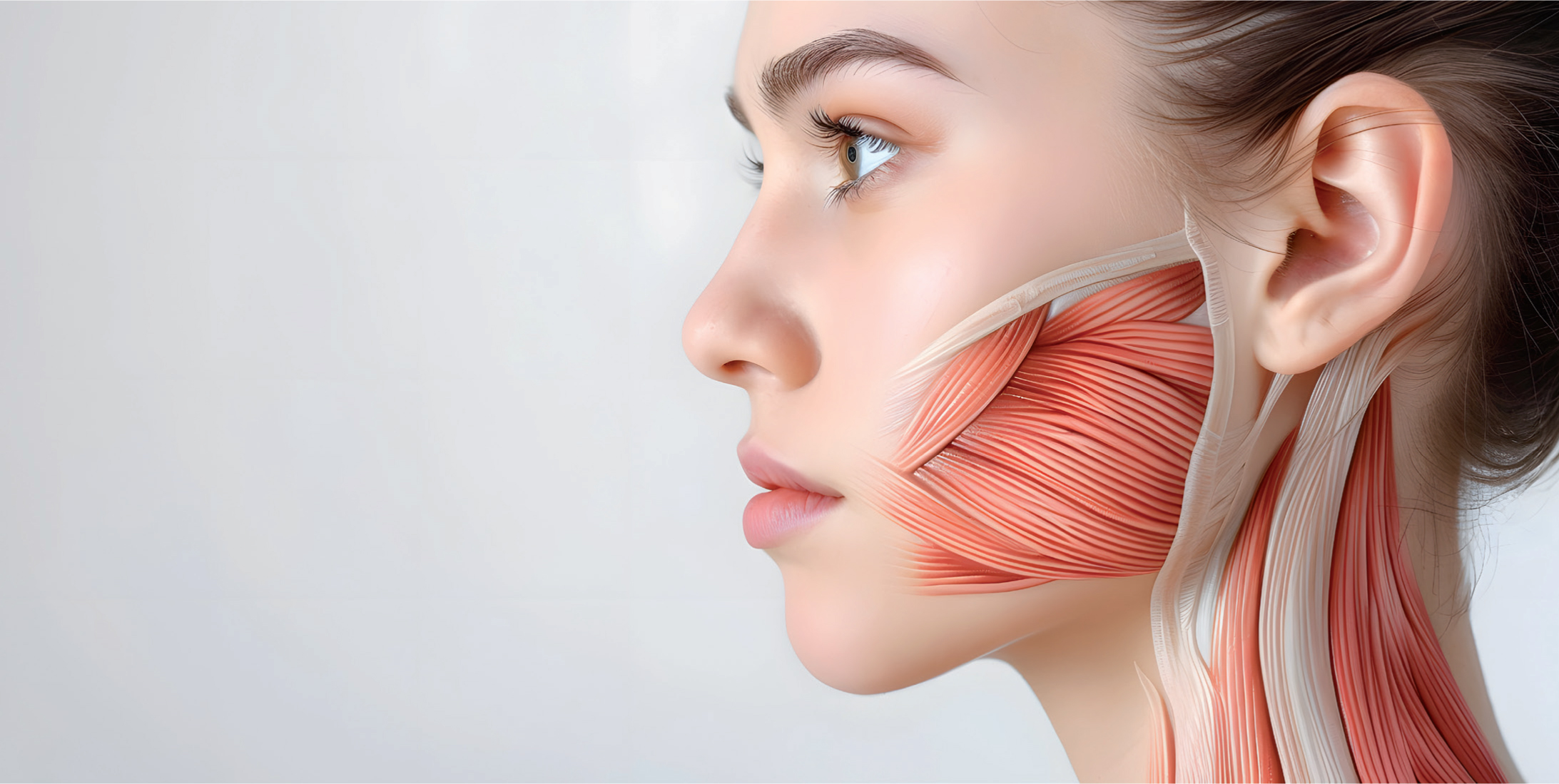
The masseter, the most prominent masticatory muscle, primarily functions to elevate the mandible against the maxilla, generating masticatory forces (Mezey et al, 2021). Beyond its crucial role in dental function, it significantly contributes to the aesthetic appearance of the lower face. Botulinum toxin type A (BoTNA) was first utilised in 1994 to address masseteric muscle hypertrophy (MMH) by inducing muscle atrophy through partial paralysis, and since then has become an effective non-surgical technique in reshaping the lower face, addressing both functional and aesthetic concerns (Kundu et al, 2022). Recognised as the standard treatment for MMH, BoTNA offers a safe, convenient solution with minimal downtime, albeit requiring multiple treatments for sustained results (Kundu et al, 2022). Studies demonstrate a high satisfaction rate of 84% attributed to effective volume reduction of the masseter, minimal complications, and the ease of the procedure (Galadari et al, 2021).
In dentistry, BoTNA has proven effective for treating temporomandibular joint dysfunction (TMJD) caused by bruxism, offering a minimally invasive alternative to surgical resection (Nayyar et al, 2014). Additionally, BoTNA relieves pain and symptoms associated with MMH and TMJD, including bruxism and associated headaches, with symptom relief beginning within four weeks, and peak efficacy observed between 8-12 weeks (Kim et al, 2023; Cheng et al, 2019; Shehri et al, 2022; Fernández-Núñez et al, 2019; Chen et al, 2023).
» Recognised as the standard treatment for MMH, BoTNA offers a safe, convenient solution with minimal downtime, albeit requiring multiple treatments for sustained results «
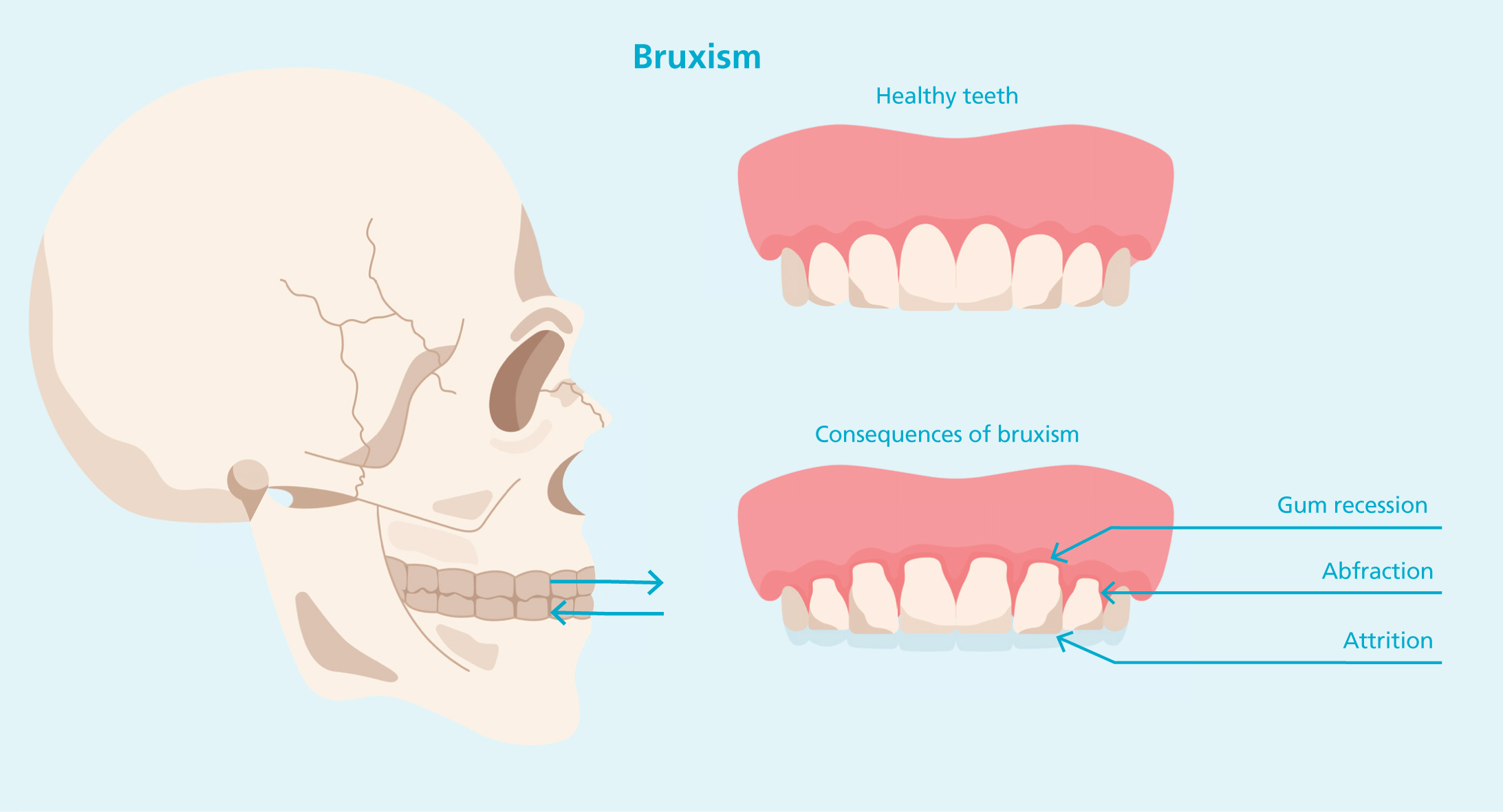
Studies demonstrate that BoTNA is effective in managing sleep bruxism by reducing the intensity of masseter muscle contractions during bruxism episodes, without reducing the frequency of rhythmic masticatory muscle activity (MMA) events (Joo Shim et al, 2020). RMMA during sleep bruxism generates substantial force – up to 66% of the maximum clenching power – contributing to masseter hypertrophy over time, providing both functional relief and cosmetic improvement (Joo Shim et al, 2020; Almukhtar and Fabi, 2019). These forces not only result in MMH but also impose excessive shear stress on the jawbones, accelerating bone resorption and ageing of the lower face (Almukhtar and Fabi, 2019; Lee et al, 2017). Other reviews demonstrate that BoTNA improves maximum mouth opening, which can significantly benefit those with severe TMJD (Delcanho et al, 2022).
Anatomy of the masseter muscle
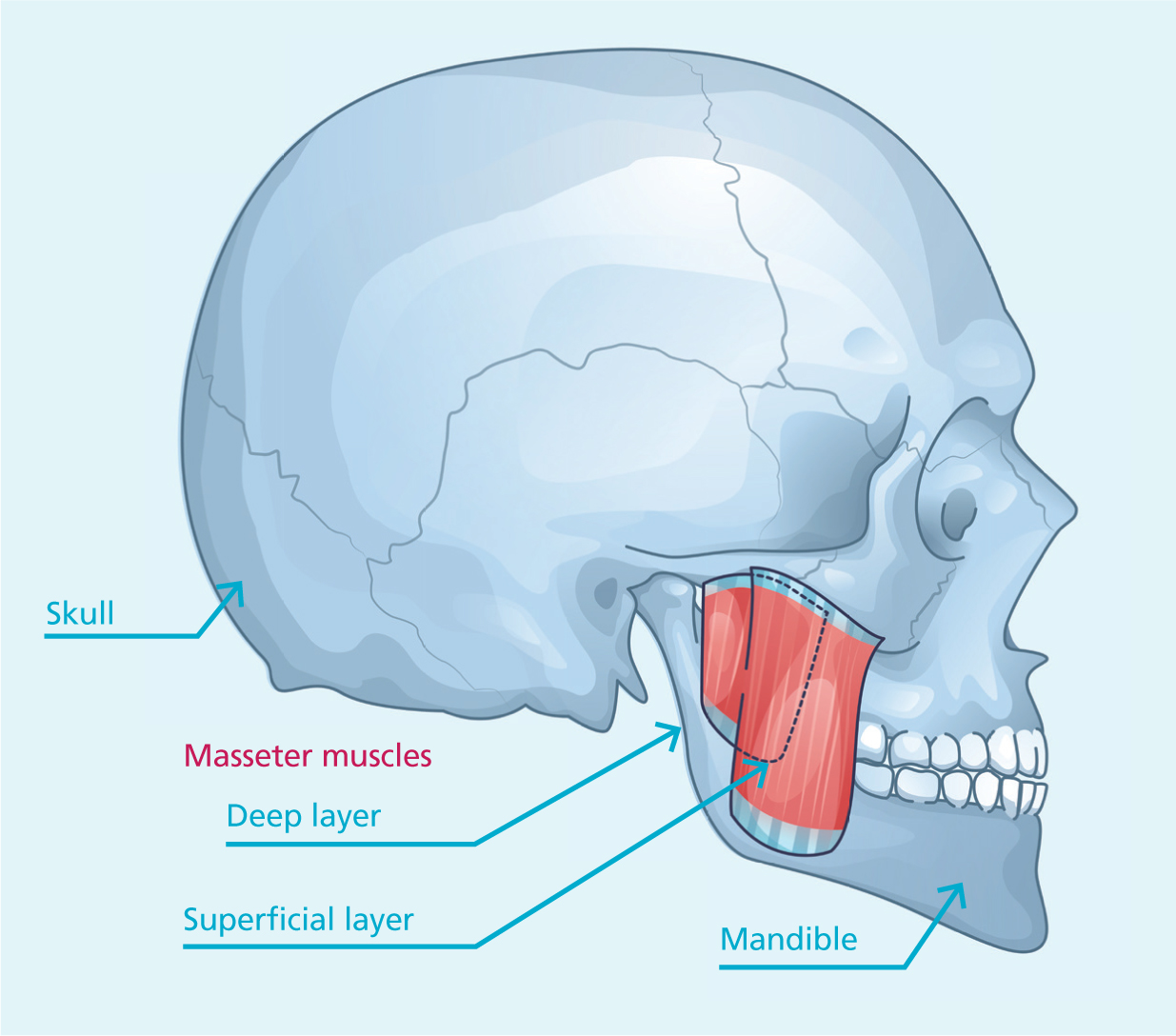
The masseter muscle has traditionally been described as quadrilateral and bilayered, comprising deep and superficial parts for mastication (Mezey et al, 2021). However, evolving research has revealed the presence of a third layer. Recent studies have refined this understanding, identifying three distinct planes of the masseter: superficial, intermediate and deep (Brunel et al, 2003; Mezey et al, 2021; Gaudy et al, 2000; Shi et al, 2024; Li et al, 2023; Kundu et al, 2022).
The superficial portion of the masseter muscle arises from the temporal process of the zygomatic bone and the lower edge of the zygomatic arch. Its fibres extend in an inferior-posterior direction, attaching to the mandibular angle and the lower part of the lateral surface of the mandibular ramus (Mezey et al, 2021). In contrast, the deep portion originates along the entire length—or the posterior two-thirds—of the zygomatic arch and inserts on the mandibular ramus, positioned superior and deeper than the superficial portion (Mezey et al, 2021). While the superficial portion covers the deep anteriorly, the posterior part of the deep section is overlaid by the parotid gland (Mezey et al, 2021). Studies found that the superficial belly is significantly thicker than the deep belly in both static and clenching states, emphasising the importance of dose distribution for effective treatment (Li et al, 2023).
The superficial layer of the masseter includes the deep inferior tendon (DIT), a crucial structure acting as a physical barrier with a hyperechoic appearance on ultrasound imaging (Hong, 2023; Popescu et al, 2024; Li et al, 2023). The DIT can be compartmentalised, transverse or longitudinal, affecting the uniform distribution of BoTNA and potentially causing paradoxical bulging (Li et al, 2023). It is classified into three types – type A, type B (B1 and B2), and type C – each with unique configurations influencing muscle segmentation and response to BoTNA, outlined in Figure 1.0 (Shi et al, 2024). A better understanding of the DIT's Structure and role can enhance injection techniques, reducing risks such as paradoxical bulging or insufficient muscle atrophy.
The masseter is innervated by the masseteric branch of the anterior trunk of the mandibular nerve (cranial nerve V3) (von Arx et al, 2018; Mezey et al, 2021). The masseteric branch innervates the lower third of the muscle, and typically corresponds to the most prominent bulging portion of the masseter during maximum contraction (Wu et al, 2023).
| Deep Inferior Tendon Type | Prevalence | Features | Impact on diffusion of BoTNA |
|---|---|---|---|
| Type A | 38.4% |
|
A more complex and irregular DIT course, making even diffusion of BoTNA challenging. |
| Type B | 54.4% |
|
Variability in thickness between the bellies of the muscle can complicate achieving balanced atrophy. |
| Type B1 | 45.6% |
|
This subtype tends to be less prone to complications as BoTNA diffusion is typically more uniform due to similar thicknesses of muscle bellies. |
| Type B2 | 8.7% |
|
This subtype presents with unique challenges due to the thin intermediate belly, which may require precise dose allocation to achieve balanced muscle atrophy. |
| Type C | 7.3% |
|
This type is more prone to uneven BoTNA distribution due to its mixed configuration, and would benefit from advanced imaging and injection techniques. |
Ultrasonography has been used to measure masseter muscle thickness variations between individuals and between genders (Kiliaridis and Kälebo, 1991). In a relaxed state, the average male and female masseter thickness was 9.7mm and 8.7mm respectively, whilst at maximum contraction, the average was 15.1mm and 13mm respectively (Kiliaridis and Kälebo, 1991; Reis Durão et al, 2017). Studies estimate the starting point of the middle part of the masseter to be approximately 2.33cm above the mandibular margin (Li et al, 2023).
Different aetiology of masseteric muscle hypertrophy
Masseter hypertrophy is commonly caused by dietary habits, chewing gum, bruxism, parafunctional habits, and stress-induced TMJ disorders and it can be classified as congenital or acquired (functional hypertrophy) (Kundu et al, 2022; Fedorowicz et al, 2013). Accurate diagnosis with a thorough patient assessment and history is crucial to distinguish it from conditions with similar appearances, such as parotid or masseter tumours, inflammatory parotid disease, salivary gland disease, and masseter intrinsic myopathy (Kundu et al, 2022).
Nocturnal bruxism is characterised by repetitive clenching or grinding of teeth during the night, leading to masseteric hypertrophy. It is estimated to impact 8-13% of the general population, with lower rates in the elderly (approximately 3%) (Beddis et al, 2018). The use of BoTNA for management of sleep bruxism demonstrates potential for minimising muscle hypertrophy, reducing grinding and protecting against dental damage (Beddis et al, 2018).
Considerations for treatment and patient selection
Ideal patients are usually those aged 18-50 years old with a broad or square lower face resulting from MMH (Kundu et al, 2022). BoTNA not only reduces muscular activity in the masseter, but also balances muscle forces to reshape facial contours without paralysing muscle function (Guida, 2024). Patients presenting with moderate-severe jowling or with pronounced cheekbones will require further considerations for suitability, and may be contraindicated for treatment as a narrower bigonial width could result in accentuated ageing features or an undesired aesthetic appearance of the lower face (Kundu et al, 2022). Masseter BoTNA treatment is particularly beneficial for patients with extroverted mandibular angles, as ultrasound-guided evaluation ensures adequate soft tissue preservation to prevent contour visibility (Li et al, 2023). Patients with thicker subcutaneous fat may experience less reduction in facial width, highlighting the importance of pre-treatment assessments (with ultrasound where possible) to tailor the approach (Li et al, 2023).
Regarding cultural considerations, facial contouring with BoTNA has become popular as a triangular-shaped lower face is considered more aesthetically pleasing amongst females, and in many Eastern cultures small, slim and oval faces are thought to bring good fortune (Ki Jung et al, 2023; Kundu, et al, 2022; Cheng et al, 2019). Patients with parafunctional habits, such as excessive grinding leading to MMH, benefit from BoTNA as it weakens the overactive masseter, reducing hypertrophy, clenching severity and bite force while lowering the risk of TMJD and dental complications (Nayyar et al, 2014; Beddis et al, 2018).
Treating masseteric muscle hypertrophy (MMH)
Historically, a surgical approach was needed. However, treatment alternatives have allowed clinicians to take on a broader range of patients. Treatment choice should be assessed on a case-by-case basis, and consider patient preference and budget (Gibson and Tatakis, 2017). The author will focus on the use of neuromodulators to treat MMH.
Neuromodulators
BoTNA injection is used to temporarily and selectively paralyse the masseter muscle through interference of the neurotransmitter system (Fedorowicz et al, 2013). Muscle paralysis is induced by BoTNA inhibiting the presynaptic release of acetylcholine at the neuromuscular junction, producing partial chemical denervation of the muscle, resulting in localised reduction in muscle activities and subsequent atrophy of the muscle (Brizuela and Ines, 2022; Mostafa, 2017; Haddadi et al, 2021; Polo, 2022; Fedorowicz et al, 2013; Wu et al, 2023). Evidence of this persistent muscular deficit post-treatment are shown through prolonged changes in muscle fibres, including atrophy and fibrosis, which may persist beyond 12 weeks (Baldwin et al, 2022).
Research has shown that multiple treatments of the masseter with BoTNA are required to obtain a stable and sustained result with significant benefits, typically repetition of the treatment every 4-6 months for 2-3 consecutive years (Graziano et al, 2016; Fedorowicz et al, 2013). The gradual muscle mass reduction over repeated sessions offers a predictable and controlled method for managing MMH (Nayyar et al, 2014; Lee et al, 2017). Studies demonstrate that BoTNA is effective for masseter-associated pain in TMJD patients, as evidenced by significant reductions in pain intensity and tender points in the masseter region over a 12-week period (Kim et al, 2023; Shehri et al, 2022). Results from CT scans before and after treatment depict dose-dependent reductions in muscle thickness, with higher doses yielding greater improvement (Cheng et al, 2019; de Souza Nobre et al, 2024; Moon et al, 2015; Delcanho et al, 2022).
The aim of BoTNA injections to the masseter muscle is decreased masticatory force, described variously as masticatory fatigue, muscle weakness, reduced bite force, and difficulty chewing hard foods, with incidence ranging from 0.9% - 63.6% (Yeh et al, 2018). This reduction begins 1-4 weeks post-treatment. Studies show a 20-40% decrease in bite force two weeks after treatment, and significant reductions in masticatory function at four weeks (Yeh at al, 2018). Bite force typically recovers within 3-8 weeks, returning to pre-injection levels around 12 weeks, however, MRI studies indicate that muscular atrophy can persist for four months, with full recovery taking six months (Yeh at al, 2018).

BoTNA is found to be particularly helpful in treating severe bruxism caseOs where traditional methods fail. Patients who have experienced persistent jaw pain and clenching despite cognitive-behavioural therapy or occlusal splints reporting significant improvements with BoTNA (Fernández-Núñez et al, 2019). Patients experienced a significant reduction in bruxism frequency and associated pain within the first four weeks after BoTNA treatment, with peak effects observed at 8-12 weeks, with the effects lasting up to 6 months (Fernández-Núñeza et al, 2019). Longer-lasting results compared to treatments for facial lines are observed due to higher doses used in masseter reduction (Wu et al, 2023).
Studies demonstrate that BoTNA not only reduces masseter thickness but also alters the expression of myosin heavy chain type II (MYH2), which has a role in muscle contraction (Moon et al, 2015). These histological changes highlight the potential for BoTNA to effectively manage conditions like bruxism and hypertrophy by targeting underlying muscular adaptations (Moon et al, 2015). Higher doses of BoTNA were associated with pronounced muscle atrophy and degenerative changes in muscle fibres, which could impact long-term functionality (Moon et al, 2015).
It is important to be aware of the potential implications of compensatory muscle overactivity when treating the masseter with BoTNA. A study demonstrated a reduction in masseteric muscle stiffness (measured with shear-wave elastography) 3 weeks post BoTNA treatment, but a consequential increase in stiffness of the temporalis muscle, another muscle of mastication (Mierzwa et al, 2022). Therefore, considerations should be made as to whether treatment of the temporalis simultaneously could contribute to the functional and/or aesthetic effect (Mierzwa et al, 2022).
Studies have demonstrated that injection of BoTNA into the masseter can also impact adjacent structures like the mandibular condyle (Dutra and Yadav, 2019). Animal studies indicate BoTNA injection induces muscle atrophy and alters loading on the TMJ, resulting in reduced mineralisation, cartilage thinning and decreased cellular activity in the mandibular condylar cartilage (MCC) (Dutra and Yadav, 2019). The findings demonstrate these effects can be observed 4-8 weeks after treatment, emphasising the importance of patient selection, treatment planning and education of the risks (Dutra and Yadav, 2019).
Contraindications
As with all BoTNA treatments, they are contraindicated in pregnancy or lactation, those with neuromuscular disorders and those with a history of hypersensitivity reactions to BoTNA or saline solution (Mostafa, 2017). It is recommended to treat with caution in patients under treatment with calcium channel blockers, cyslosporine and aminoglycosides (Mostafa, 2017). It is advised that treatment is not repeated before the effect has completely faded in order to reduce the risk of antibody formation against the toxins (Mostafa, 2017).
Technique and anatomy
Common techniques range from a 2-point, 3-point to 5-point injections, with different methods described to avoid complications such as muscle asymmetry, inadvertent muscle paralysis, parotid herniation and paradoxical bulging (Kundu et al, 2022; Hong, 2023). Key injection sites must account for individual anatomical variations, such as baseline asymmetry and underlying muscle bulk (Guida, 2024; Ng and Yang, 2021). Most literature describes treating within a safety zone of the masseter to reduce side effects (Kundu et al, 2022; Ng and Yang, 2021). Advanced techniques utilise ultrasound guidance to increase injection precision and reduce risks like paradoxical bulging (Kundu et al, 2022; Popescu et al, 2024; Shi et al, 2024). The use of ultrasound-guided injections has been highlighted recently to assist in successfully treating those with differing masseteric DIT patterns with BoTNA as practitioners can obtain more even distribution of BoTNA by identifying ideal injection sites and depths, particularly for complex configurations such as type B2 or type C, where variations in muscle belly thickness necessitate precise dose adjustments (Shi et al, 2024). For patients suffering with pain associated with MMH, BoTNA injections are administered to specific tender points identified through palpation, with the dosaging tailored to patient needs (Kim, 2023). This approach ensures effective pain relief and muscle relaxation without compromising function (Kim et al, 2023; Alkhouri et al, 2022). This has been confirmed by significant decreases in electromyography (EMG) readings and pain perception scores where patients experienced improved quality of life, reduced jaw discomfort and less sleep bruxism (Shehri et al, 2022).
The resulting effect usually becomes apparent within the first 2-6 weeks, reaching peak result at 3 months, lasting an average of 6 months and repeat injections necessitated every 4-7 months for sustained improvement and reduction in MMH (Kundu et al, 2022). Studies have demonstrated that there is approximately a 30% reduction in masseter volume, observed within 2-4 weeks post-injection, with effects lasting up to 12 weeks, depending on dosage (Galadari et al, 2021). Further EMG studies highlight that BoTNA-induced paralysis significantly reduces masseter muscle activity within 4 weeks, with partial recovery by 12 weeks (Baldwin, et al, 2022; Joo Shim et al, 2020). However, certain muscle fibres fail to regain normal function, resulting in persistent atrophy (Baldwin et al, 2022). Prolonged denervation may lead to structural changes, such as collagen deposition and fibre degeneration, contributing to functional impairments in the treated muscle (Baldwin et al, 2022). The longevity of BoTNA effectiveness is not related to dose but depends on frequency of muscles' mobility and the degree of hypertrophy.
A simplified technique for administering BoTNA has shown effective results in managing muscle hypertrophy using 3 injection points (Andrade and Deshpande, 2011). Apex point (point A) describes the maximal swelling during clenching, and points B and C are located in less prominent hypertrophic regions of the muscle, with the toxin being injected deeply within the muscle (Andrade and Deshpande, 2011). Ultrasonographic follow-up assessments revealed that three months post-treatment there were significant reductions in muscle thickness (Andrade and Deshpande, 2011).
Research has demonstrated that trace elements such as zinc can significantly enhance the effectiveness and longevity of BoTNA (Elgendy et al, 2024). Zinc supplementation was found to increase BoTNA action by maintaining the toxin's biological activity and supporting its role as a zinc-dependent metalloprotease, which resulted in greater masseter muscle fibre atrophy (Elgendy et al, 2024). On the contrary, copper supplementation reduced BoTNA efficacy, which mitigated masseter muscle degradation and promoted muscle fibre recovery (Elgendy et al, 2024).
Interestingly, a study demonstrated that increasing masticatory movements post-treatment can enhance the duration of the therapeutic effects of BoTNA for masseter hypertrophy (Wei et al, 2015). Purposeful strengthening of masticatory effort (e.g. by chewing gum) during the muscle's denervated atrophic stage has been shown to delay re-hypertrophy, extending the treatment's effectiveness (Wei et al, 2015). Further studies and a deeper understanding are required to strengthen the concept and establish clear post-treatment protocols to follow to optimise the benefits and longevity of BoTNA.
Dose
Dosage recommendations vary widely, from 20-140 units per side, depending on patient needs, history of treatment and degree of MMH, which can vary between cultures (Kundu et al, 2022). Studies reporting a significant reduction in masseter muscle thickness 8-weeks post-injection involved patients receiving 35 units of onabotulinumtoxinA per side to show the most pronounced improvement (Cheng et al, 2019). Satisfaction rates were highest in the high-dose group, demonstrating the importance of tailoring dosage to achieve both functional and aesthetic goals (Cheng et al, 2019). Interestingly to note, the lowest dose (15 units per side of onabotulinumtoxinA) showed measurable improvements also, highlighting the efficacy of BoTNA at varying dosages (Cheng et al, 2019). Another study used 75 units of abobotulinumtoxinA per side, divided into 3 injection points, using ultrasound to measure precise muscle thickness and guide accurate injection placement (de Souza Nobre et al, 2024). This study observed a reduction in masseter muscle thickness within one month, with the most pronounced effects appearing at the 6-month follow-up following a re-treatment (de Souza Nobre et al, 2024). A recommended dose distribution of 53.23% to the superficial belly and 46.77% to the deep belly ensures balanced muscle atrophy and reduces risk of over-treatment (Li et al, 2023).

An additional study compared BoTNA in varying doses, typically between 20-80IU per masseter muscle, distributed along multiple injection points (Fernández-Núñez et al, 2019). Injections were placed at three strategic points in the masseter to maximise therapeutic outcomes without compromising functionality (Fernández-Núñez et al, 2019). The systematic review concluded that BoTNA is a safe treatment option when doses remain below 100IU per session (Fernández-Núñez et al, 2019).
Complications
BoTNA masseter injections are widely regarded as safe and effective when administered with an appropriate technique and dose, offering good results with minimal risks. Localised side effects such as pain, bruising, inflammation, infection, insufficient muscle strength loss, nerve palsy, and haematoma, typically resolve within 7-14 days (Mostafa, 2017; Yeh et al, 2018). Improper injection technique can result in paradoxical bulging, changes in facial expression, asymmetrical smile, difficulties in speech, eating and/or drinking (Mostafa, 2017; Haddadi et al, 2021; Yeh et al, 2018). Cadaveric studies reveal that the Risorius muscle attaches to the middle or anterior part of the masseter in over 95% of individuals, making it susceptible to inadvertent paralysis or BoTNA diffusion-related complications, resulting in smile restriction or asymmetry (Yeh et al, 2018; Bae et al, 2014). These undesired changes, including loss of dimples, have varied incidence in studies, occurring between 0.15%-27.3% of cases, appearing within 2-4 weeks post injection and resolving in 1-2 months (Yeh et al, 2018; Kundu et al, 2022).
Poor effect, or lack of, is due to insufficient dosage or superficial BoTNA placement, with post-treatment asymmetry being a result of failure to identify the patient's anatomical difference of masseter size, with an incidence rate of 2.5% (Yeh, 2018). Muscular bulging whilst masticating is known as paradoxical masseteric bulging (PMB), with an incidence rate of 0.49%-18.8% (Yeh et al, 2018). PMB is a complication closely linked to the DIT structure, where inadequate diffusion of BoTNA leads to uneven muscle paralysis (Shi et al, 2024). This results in excessive compensatory contraction of untreated superficial masseter muscle fibres, which, if does not recover in 2 weeks post treatment, should be treated with a more superficial additional BoTNA dose to the masseter (Yeh et al, 2018; Hong, 2023). Use of ultrasound guided injection can reduce the incidence of this occurring by providing real-time imaging of the masseter structure (Popescu et al, 2024; Shi et al, 2024). Translating the visual structures into precise needle placement can be challenging, and so whilst ultrasound improves precision, there is a higher level of skill and experience required to accurately replicate the desired placement in the observed muscle (Popescu et al, 2024). The biphasic injection technique using BoTNA describes a method of treating MMH by treating both the superficial and deep layers of the masseter muscle, achieving a more uniform muscle reduction (Chirico et al, 2021).
Sunken cheeks, characterised by volume loss in the mid-face, have an incidence rate of 0.44%-26.5% and typically recover in 8 weeks (Yeh et al, 2018). It may be linked to high injection positions, and zygomatic prominence seen post-treatment might be a secondary effect; to avoid this, lower masseter muscle injections are recommended (Yeh et al, 2018; Hong, 2023). Sunken temporal fossa is a rare side effect, with an incidence of about 0.05%, appearing approximately a month after treatment, which may result from atrophy of the temporalis muscle or the downward displacement of fat pads due to reduced masseter muscle volume (Yeh et al 2018; Kundu et al, 2022).
The majority of complications associated with BoTNA injection into the masseters appear within 2-4 weeks of the treatment, and disappear within 12 weeks (Yeh et al, 2018; Kundu et al, 2022). Despite the transient nature, reducing the likelihood of complications will improve patient satisfaction and confidence in their medical practitioner. To minimise these risks in the masseter region, practitioners should have a deep understanding of the anatomy in this region, maintain a safe injection zone (at least 1cm from the anterior border of the masseter) and use a correct injection technique (i.e. predominantly deep injections), taking consideration of the author's described safety technique.
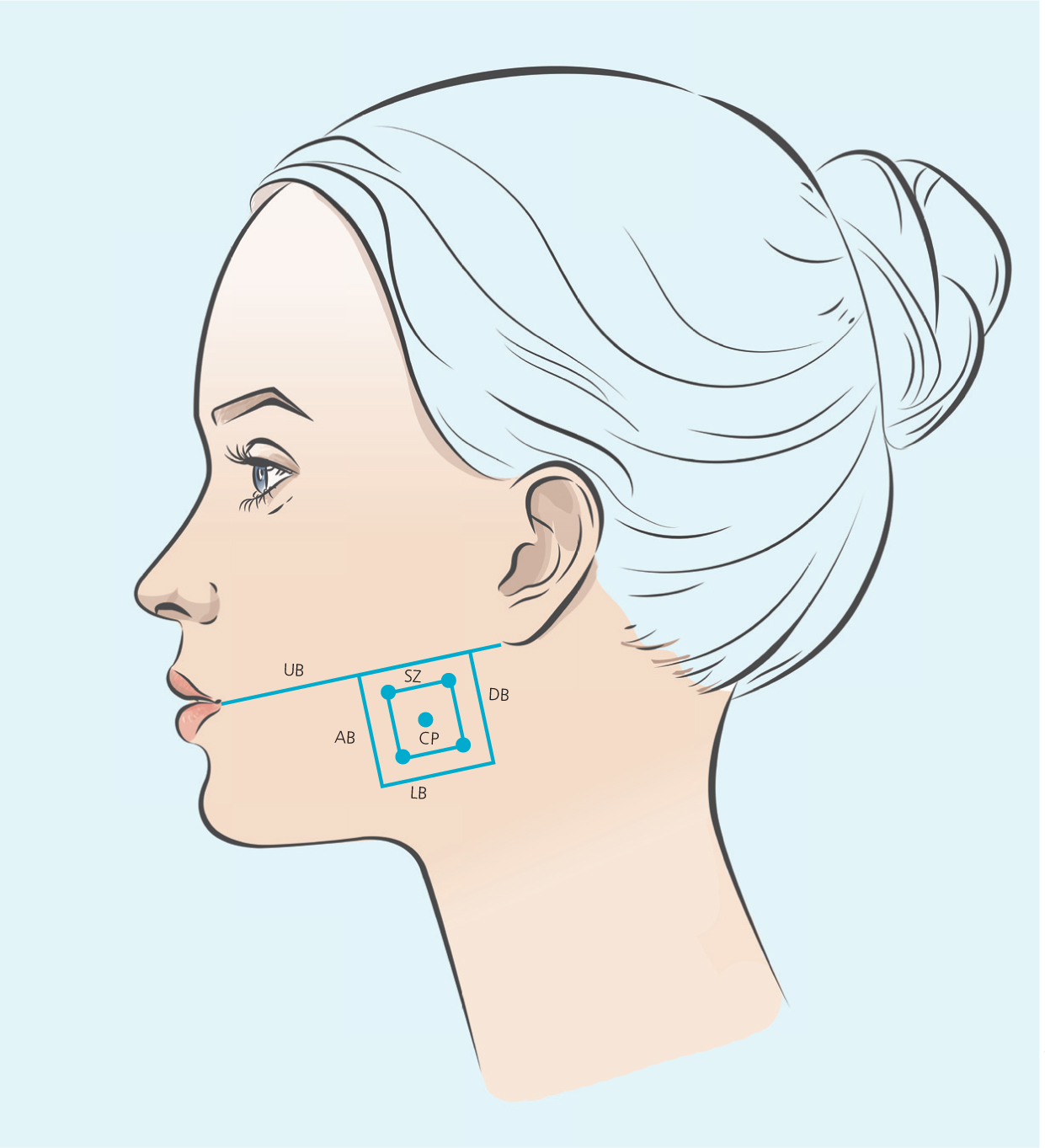
Injection technique – masseter safety frame injection technique - MSFIT
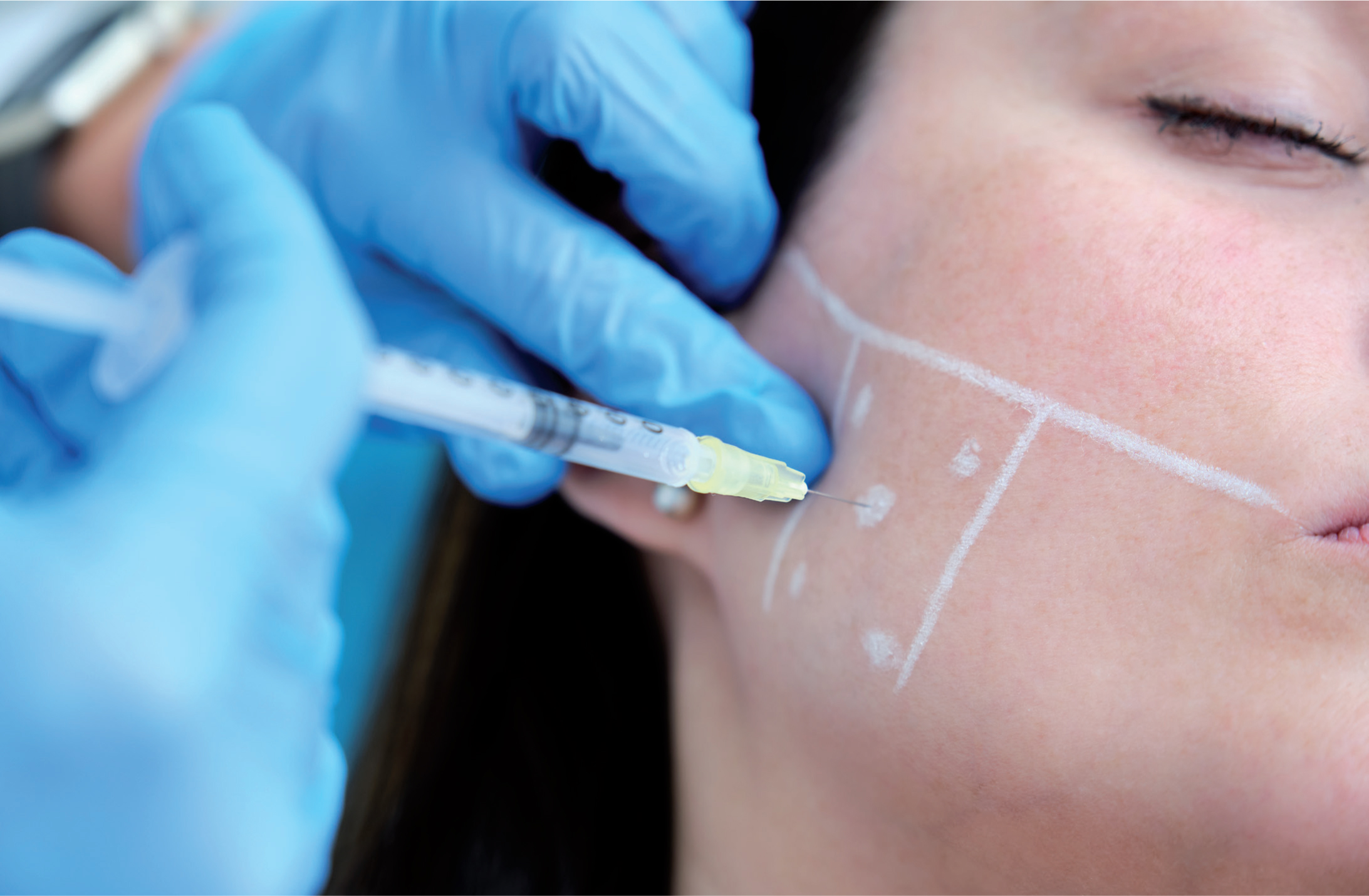
The author has devised a technique for marking up the masseter to improve safety, minimise risk of complications, ensure efficacy of treatment and allow reproducibility, with consideration of patient anatomical variations. Named the Masseter Safety Frame Injection Technique (MSFIT), the method involves using an aesthetic pen marker, with the patient sat upright, to demarcate a 1cm frame ‘safety zone’ within the bulk of the masseter muscle, with centrally-angled injections to a precise depth, allowing reduced risk of inadvertent paralysis of the risorius muscle, whilst confining the dosage to the prominence of the masseter muscle to obtain successful outcomes. The central point is the most pronounced point of the masseter muscle during occlusion, which can be assessed by asking the patient to activate the muscle through maximum clenching of the teeth, and visualising the greatest protrusion of the masseter muscle from a frontal view.
This anatomically guided safety frame provides a clear method for treating MMH with BoTNA and for educational purposes. Adjustments may be needed for milder MMH or narrower profiles, and incorporating ultrasound guidance with MSFIT can further enhance precision by identifying the DIT pattern.
5 Points of injection will be delineated – the inner most CP, and each 4 corners of the ‘inner safety zone’.
Before injection, BoTNA is diluted to produce a resulting dose of 4 units per 0.1ml by adding 2.5ml 0.9% bacteriostatic saline solution to 100u of vacuum-dried clostridium botulinum toxin type A. Insulin syringes of 0.5ml with a 29g (12.5mm length) needle were used in order to reach the desired depth. Using an assessment of masseteric hypertrophy (and categorising the severity on palpation of maximum muscular contraction), the injection sites were marked with an aesthetic pen marker, applying the MSFIT technique, an aseptic cleanse of the area was carried out, and 20U of BoTNA (OnabotulinumtoxinA, Botox®) was equally administered over the 5 described points (4U per point) per side for a total dose of 40U.
Conclusion
Botulinum toxin type A (BoTNA) effectively addresses functional and aesthetic concerns in MMH. Understanding masseter anatomy, including the deep inferior tendon (DIT), is critical to achieving successful and uniform results while minimising complications such as paradoxical bulging or muscle asymmetry. Techniques using a guided injection technique, such as the masseter safety frame injection technique (MSFIT), and the use of ultrasound, further enhance the precision and safety of injections, offering tailored solutions for complex patient cases.
BoTNA's dual role in functional improvement and aesthetic contouring makes it a versatile and invaluable tool in aesthetic medicine. However, as this is currently an off-label treatment, practitioners are strongly encouraged to pursue advanced in-person training to ensure patient safety, optimal results, and adherence to best practices.
With ongoing advancements in technology such as ultrasound guidance, and developments in understanding, as well as injection techniques and strategies to optimise toxin diffusion, BoTNA will continue to remain a pivotal treatment in both aesthetic and functional management of masseteric muscle hypertrophy.



