



Augmentation mammoplasty is commonly performed by inserting silicone or saline implants, which requires general anaesthesia. Dermal fillers also can be used as an alternative to implants as a less invasive procedure for breast augmentation and using only local anesthesia. AQUAfilling gel is a hydrophilic gel composed of 98% sodium chloride solution (0.9%) and 2% cation copolyamide (Amin et al, 2004; Jung et al, 2018). Although less well-known in the UK, it is one of the dermal fillers that can be used in other countries, such as Turkey; however, the US Food and Drug Administration (FDA) has not approved the usage of this gel (FDA, 2015). Furthermore, there are several complications of this filler in the literature (Arslan et al, 2017; Jung et al, 2018; Son et al, 2018).
This case report describes multimodality imaging features of breast augmentation using AQUAfilling gel injection without complication. The authors report that the filling material causes difficulty in radiological imaging.
Case report
A 40-year-old woman who had undergone breast augmentation via AQUAfilling gel injection 2 years ago was referred to the three of the authors' radiology department due to palpable mass on the upper outer quadrant of the right breast. Mammography revealed a bilateral lobulated isodense mass in the retroglandular area (Figure 1). There were no significant radiological findings to meet the palpable mass on the ultrasound. The ultrasound showed a bilateral hypoechoic fluid collection with internal echoes between fibroglandular tissue and fascia of the pectoralis muscle (Figure 2). Breast magnetic resonance imaging (MRI) was carried out, but there was no finding in the breast MRI to explain the palpable mass. An annual follow-up was suggested, considering that the finding was related to breast augmentation. AQUAfilling gel injection was hypointense in T1-weighted images (Figure 3) and hyperintense in T2-weighted images (Figure 4). In the subtraction contrast-enhanced T1-weighted dynamic series, there was no enhancement of filling material. Diffusion-weighted images showed that ADC values of filling material was 2.4 mm2/sec x 10-3 on 3-T MRI with 2 b values (b = 50, 800 s/mm2). In this case, there was no suggestion of migration complication, as there was no scattering material surrounding the tissues.
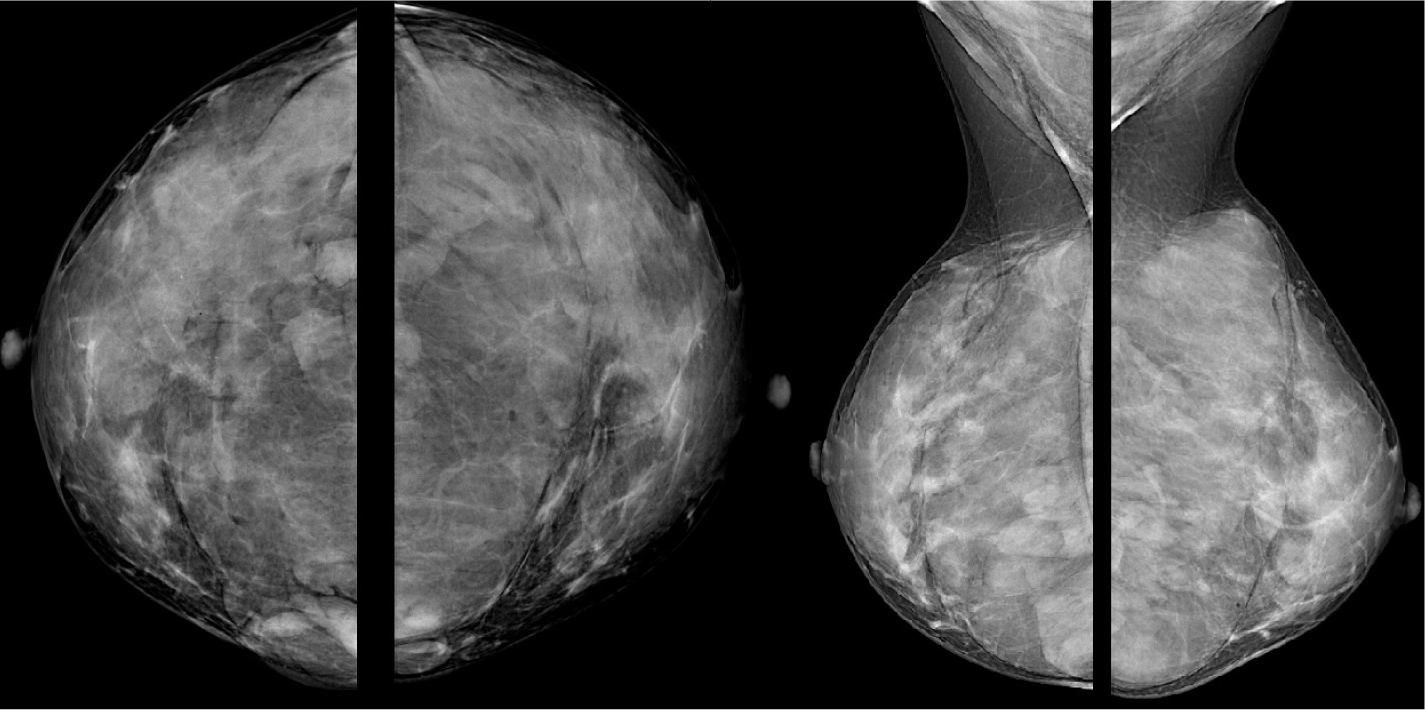 Figure 1. Mammography shows a bilateral lobulated isodense mass in the retroglandular area
Figure 1. Mammography shows a bilateral lobulated isodense mass in the retroglandular area 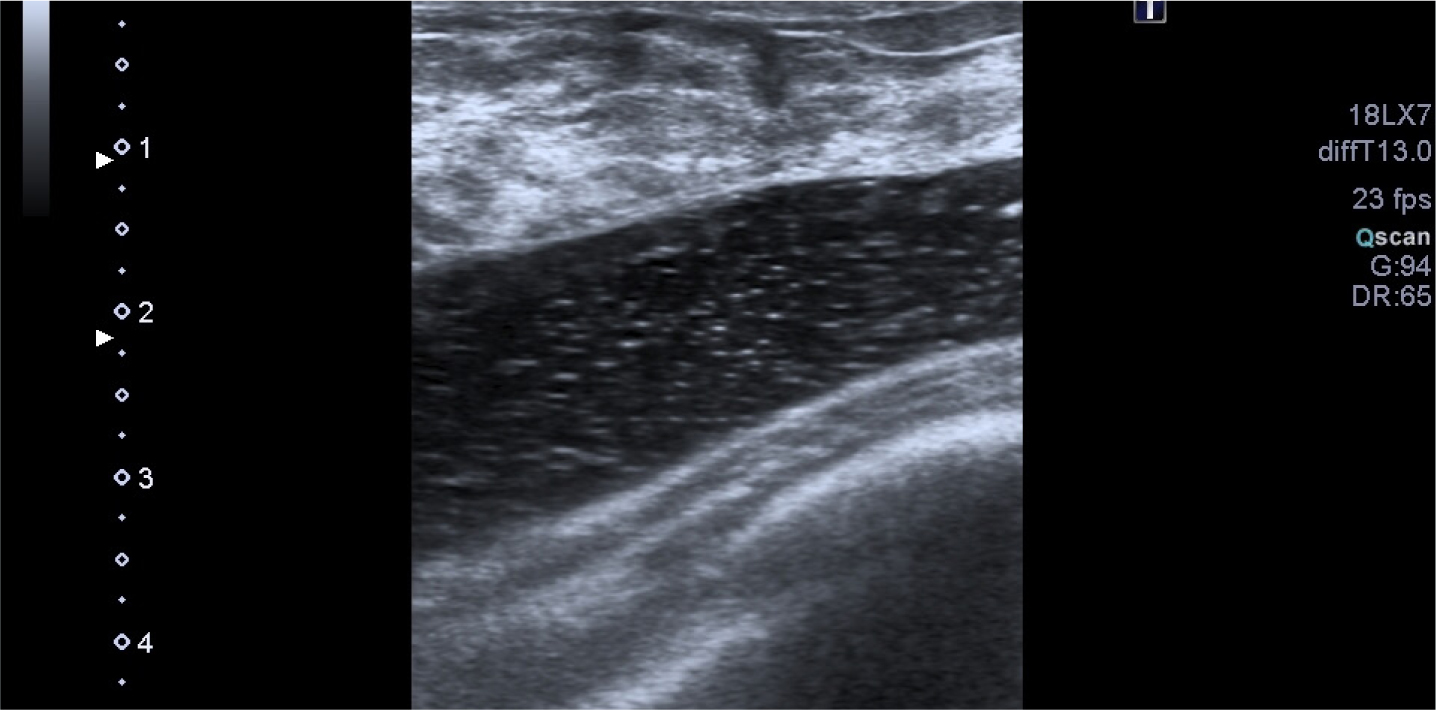 Figure 2. Hypoechoic fluid collection with internal echoes between fibroglandular tissue and fascia of the pectoralis muscle
Figure 2. Hypoechoic fluid collection with internal echoes between fibroglandular tissue and fascia of the pectoralis muscle 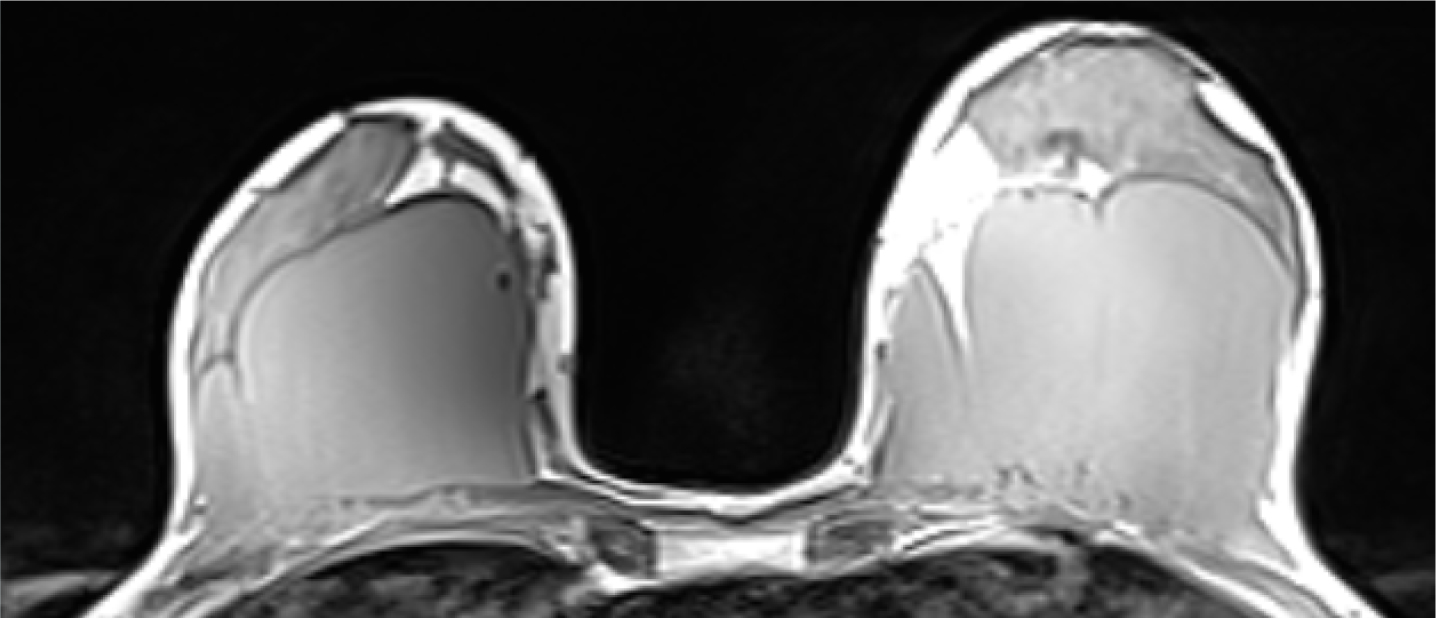 Figure 3. Bilateral hypointense mass between the retro-fibro glandular area and the pectoralis muscle
Figure 3. Bilateral hypointense mass between the retro-fibro glandular area and the pectoralis muscle 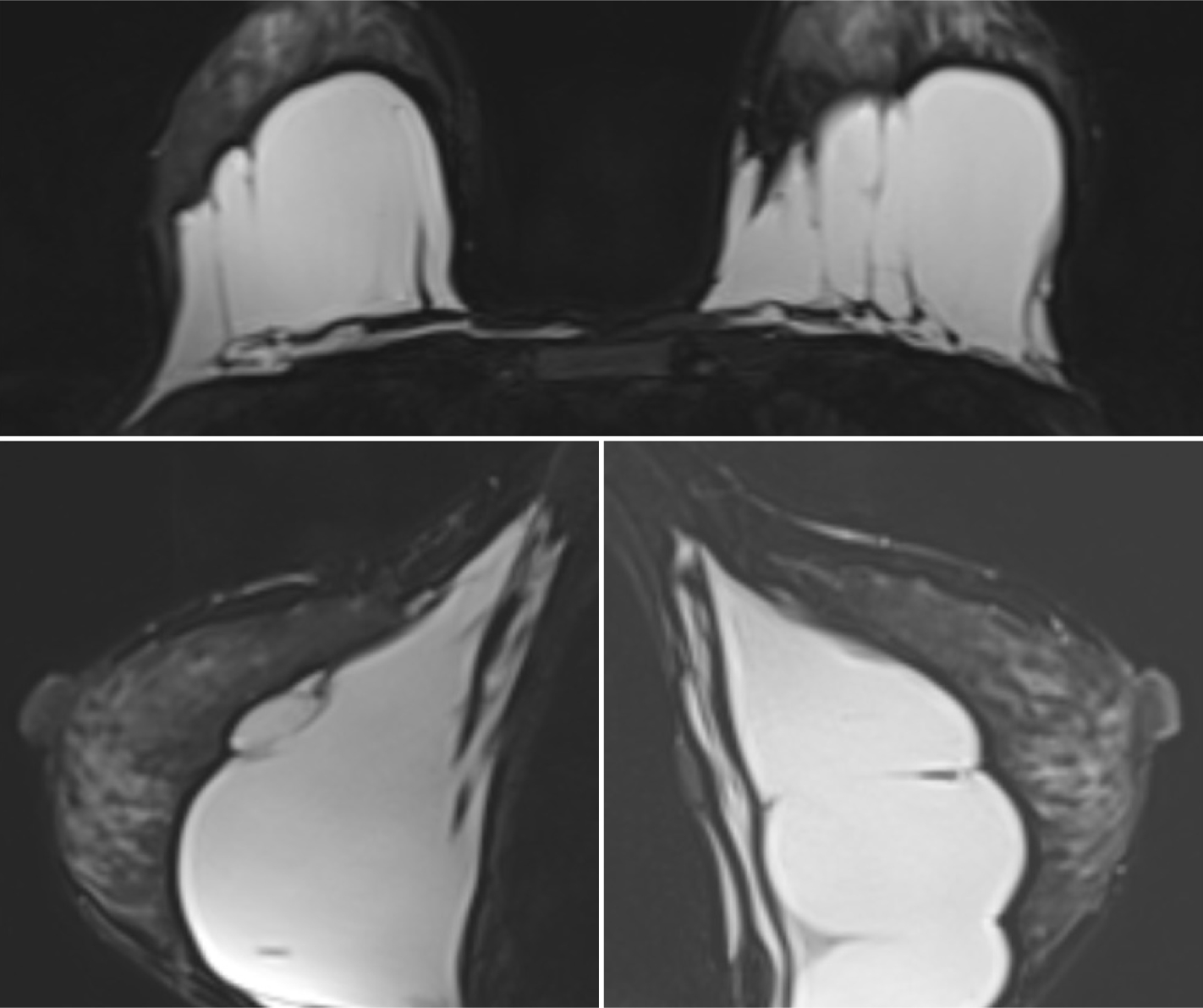 Figure 4. Gel is seen as lobulated hyperintense masses on T2-W image
Figure 4. Gel is seen as lobulated hyperintense masses on T2-W image
Discussion
AQUAfilling gel is one of the dermal fillers used in breast augmentation with local anaesthesia. There are several cases of complications of this procedure (Arslan et al, 2017; Jung et al, 2018; Son et al, 2018). The FDA has not approved the use of this gel, and there are some concerns about its safety (FDA, 2015; Jung et al, 2018). The imaging findings of AQUAfilling gel are similar to those of polyacrylamide gel (Teo and Wang, 2008). Furthermore, Son et al (2018) described the mammographic and sonographic features of this gel. To the authors' knowledge, no reports have described MRI findings of AQUAfilling gel breast augmentation without any complication. In this case, there is no evidence of complications following this procedure, both clinically and mammographically; however, it necessitated additional imaging modalities of an ultrasound and MRI. Superpositions caused by the injected material complicate mammographic evaluation. The MRI showed lobulated fluid collections with septum between fibroglandular tissue end pectoralis muscle.
Besides complications such as infection, localised lumps or concerns about its toxicity and irritation due to migration from the AQUAfilling gel, the difficulty in breast imaging is another aspect that should be considered when proposing the use of AQUAfilling gel for breast augmentation.



