







The ageing process occurs through biological, biochemical and molecular mechanisms, which sometimes overlap. Qualitative and quantitative changes in the extracellular matrix proteins occur, and, consequently, there is a reduction in the skin's retraction capacity and tensor power, resulting in increased fragility and the formation of wrinkles. Additionally, collagen cells and elastic fibres are in lesser quantities, leading to decreased skin elasticity. Combined with these factors, there is extrinsic ageing, mainly due to chronic sun exposure (photoageing) inducing important skin changes (Zouboulis and Boschnakow, 2001; Hirata et al, 2004).
There are different therapeutic options for the treatment of this condition, including intradermotherapy, also called mesotherapy, which is considered a non-surgical and minimally invasive procedure, where diluted pharmacological substances are applied, both intradermally and subcutaneously on several locations in the area to be treated. Thus, the dermis then becomes a reservoir in which the products activate dermal receptors and diffuse slowly, using the microcirculatory pathway. Applications can be made weekly or monthly, and the number of sessions reported in previous studies can vary from four to 10 applications (Herreros et al, 2011; Oliveira et al, 2013; Moura Filho et al, 2017).
Oliveira et al's study (2013) evaluated intradermotherapy as an additional therapeutic option for the treatment of photodamage in 30 female patients, with five applications of injectable polyrevitalising agents on the face. It was observed that the supply of antioxidants (vitamins C and E and glutathione), tissue nutrients in the form of vitamins (C, E and the B complex), coenzymes, nucleic acids, amino acids and hyaluronic acid without crosslinking was effective for improving dermal thickness and fibre reorganisation, leading to an improvement in light to moderate photodamage signals.
However, there are still few studies on the use of effective formulations for the facial rejuvenation treatment using the technique of intradermotherapy with conventional injection systems that demonstrate consistent clinical results. Therefore, this study aimed to investigate the effects of intradermotherapy with conventional injection using an anti-ageing substance in the treatment of facial rejuvenation in women, also determining its clinical results.
Materials and methods
The study is a randomised clinical trial. The research was approved by the Ethics Committee of the Federal University of Rio Grande do Norte (code: 3.948.777), Natal, Brazil, and was conducted in accordance with the recommendations of the Consolidated Standards of Reporting Trials (CONSORT). All volunteers signed an informed consent form before the study began.
The group allocation sequence was followed according to a list generated by the Software Research Randomizer, and the volunteers were allocated according to the sequence in which they were evaluated. The intervention was tested in comparison with the control group and among the volunteers in the treated group, using the analysis of independent groups to control the study.
Participants
Initially, 24 volunteers who showed signs of facial skin ageing, chosen in a non-probabilistic manner in the city of Natal, Brazil, were evaluated. The inclusion criteria were women aged 45 to 60 years who presented signs of facial skin ageing (tissue flaccidity, wrinkles, elastosis, etc); Fitzpatrick skin type II or III; and who could not be having other aesthetic treatments for rejuvenation, with preserved local sensitivity and the capacity for understanding.
The exclusion criteria were applied to all volunteers who showed changes in sensitivity and who presented contraindications regarding the use of the therapeutic resource and substances used in the research, which are: collagen-related diseases, such as keloids, healing and/or proteins synthesis problems; rejection of any component of the substances to be used; being in recovery from facial cosmetic surgery; and/or using anti-inflammatory drugs up to 1 week before the start of the study. If any of the volunteers needed to take medication within 1 month after the beginning of the study, they would be discarded from the sample. Participants who did not agree with the procedures, presented sensitivity disorder during therapy or did not adapt to the times and procedures were discontinued from the research.
As a result, there was a sample loss of 6 volunteers during the protocol, leaving 18 participants. These were divided into two groups: group 1 (G1) (intradermotherapy with 12 volunteers) and group 2 (G2) (control with 6 volunteers). The average age of the volunteers was 56.7 years.
Evaluation procedures
After the selection, the participants were informed about the procedures to be performed and those who were in agreement signed the informed consent form. All participants underwent a facial evaluation, which was performed pre-treatment and 45 and 90 days after the beginning of the applications. The Facial Assessment Protocol (PAF) (Micussi et al, 2008) was used as an instrument for data collection in this research, where identification, medical history, functional clinical examination, including inspection and identifying Fitzpatrick skin type and wrinkle classification by using Lapière and Pierard (1987), Tsuji (1986) and Glogau's (2021) scales, were addressed.
The photos were taken with a semi-professional camera (Canon, SX530 HS, Japan), with a resolution of 16 MP. The volunteers were positioned seated, and the records were made in the anterior and lateral view (right and left) using a support tripod positioned at a height of 66 cm from the floor and placed 55 cm away from the volunteer, for better viewing and standardisation of the photos.
The volunteers underwent the substance allergy predictive test 2 days before the treatment started. For this, they received a 0.3 ml injection of the active substance intradermally to identify possible episodes of allergic reaction/irritation to the product, such as hyperaemia, itching, tingling or oedema. However, no volunteer presented such reactions, and they only reported that the region felt sore after the test.
Upon completing the established protocol, the volunteers answered the questionnaires for patient satisfaction analyses (Segot-Chicq et al, 2007), and the Global Aesthetic Improvement Scale (GAIS) by Narins et al (2003), through which it was possible to classify the treatment responses, allowing for comparison at different times after the therapeutic intervention.
Treatment
The procedures were performed with Lebel needles (32 G, 4 mm), Smart Gr brand, an anti-ageing substance with TGF-β3 nanofactor, EGF nanofactor, silicon and hyaluronic acid from low molecular weight, manufactured by Mezzo Dermocosméticos (São Paulo, Brazil) and injectable saline of the Eurofarma brand. During the sessions, all volunteers were placed in the supine position, and the treatment was applied to the facial region.
In the intradermotherapy group (G1), the anti-ageing substance was applied, while in the control group (G2), the application was performed only with injectable saline solution. The treated areas were the frontal region, where injections were applied to 10–18 points; orbicularis of the eye, with 3–6 points; the zygomatic area, with 3–5 points; and the nasogenian groove, with 2–4 points (Figure 1).
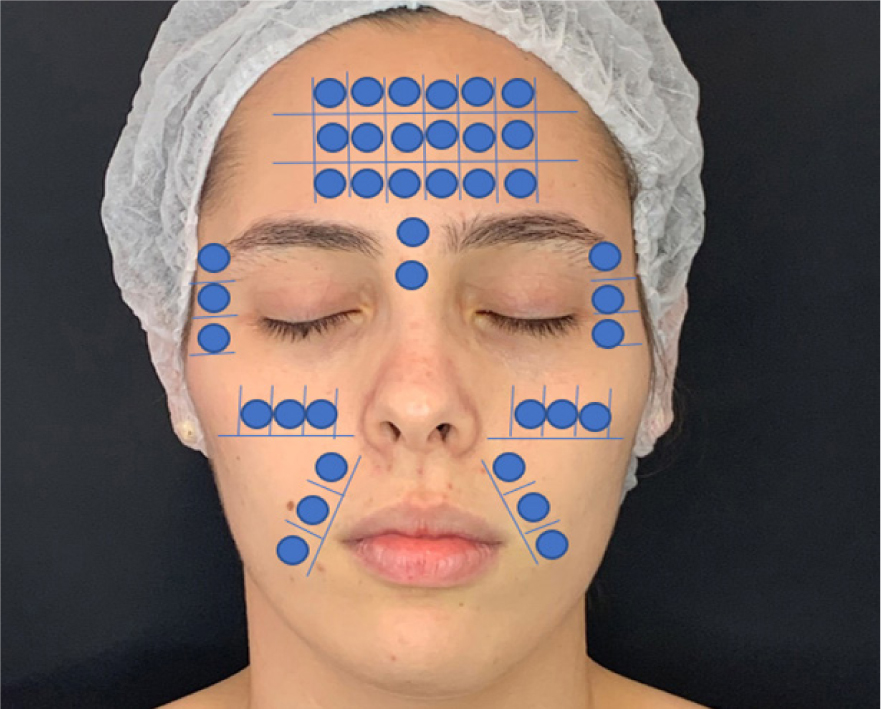 Figure 1. Estimated substance injection points for treatment
Figure 1. Estimated substance injection points for treatment
In both groups, before the beginning of the procedures, the facial region received an antiseptic cleaning with a gauze soaked in 70% alcohol, then the points for injection were marked and the injection of active ingredients or saline was performed using intradermal microinjections, with needles positioned at a 45° inclination in relation to the skin (intradermal technique). Into each point, 0.1 ml of the anti-ageing or physiological component was injected and, to alleviate the soreness, 1 ml of lidocaine without 2% vasoconstrictor was added to each 4 ml of the solution. Four treatment sessions were carried out at 15-day intervals. Each treatment session lasted approximately 30 minutes.
Data analysis
The descriptive and inferential statistics of the data were performed using the Statistical Package for the Social Science (SPSS) (version 24.0) programme. The normality of the data was monitored by the Kolmogorov-Smirnov (KS) test. The one-way analysis of variance (ANOVA) test was applied for intergroup comparison for the comparison between the groups, the data for which are parametric. The level of significance was set at 5% (p <0.05). Data collection and correlation were presented in tables and figures.
Results
The results obtained using the PAF are described in Table 1 in absolute values (%). Regarding the characteristics of the volunteers, there was a predominance of Fitzpatrick skin type II and III. There was a predominance of static wrinkles and sagging, with signs of advanced photoageing (Glogau type III) (Glogau, 2021) in both groups. Using the Tsuji classification (Tsuji et al, 1986), the treated group presented a higher classification of superficial wrinkles and scored Grade I (expression wrinkles) using the Lapière and Pierard classification (Lapière and Pierard, 1987), while the control group presented deep wrinkles in the Tsuji classification and Grade II (dermoepidermal thinning) from the Lapière and Pierard classification (Lapière and Pierard, 1987).
Table 1. Facial assessment data using the Facial Assessment Protocol
| Evaluation | Result (%) | ||
|---|---|---|---|
| Group 1 | Group 2 | ||
| Skin type | Eudermic | 19% | 0% |
| Alipic | 25% | 0% | |
| Mixed | 44% | 83% | |
| Oily | 13% | 17% | |
| Glogau Wrinkle Scale (Glogau, 2021) | Type I | 0% | 17% |
| Type II | 19% | 33% | |
| Type III | 81% | 50% | |
| Phototype | Type I | 13% | 0% |
| Type II | 56% | 17% | |
| Type III | 19% | 67% | |
| Type IV | 13% | 17% | |
| Wrinkle type | Static | 81% | 83% |
| Dynamic | 19% | 17% | |
| Flaccidity | Yes | 69% | 100% |
| No | 31% | 0% | |
| Tsuji classification (Tsuji et al, 1986) | Superficial | 69% | 17% |
| Deep | 31% | 83% | |
| Lapière and Pierard classification (Lapière and Pierard, 1987) | Grade I | 63% | 17% |
| Grade II | 31% | 67% | |
| Grade III | 6% | 17% | |
In the photographic analysis, it was possible to observe that the group treated with the anti-ageing substance (G1) showed a more significant reduction in the nasogenian groove (Figure 2) after two sessions (45 days after the beginning of the treatment), and less deep wrinkles were also noticed in the perioral area (Figure 3) after the four treatment sessions, with the evaluation performed 90 days after the beginning of the treatment. For the group that received only the saline solution (G2), no notable differences were observed in any of the angles in the evaluated periods (Figures 4 and 5).
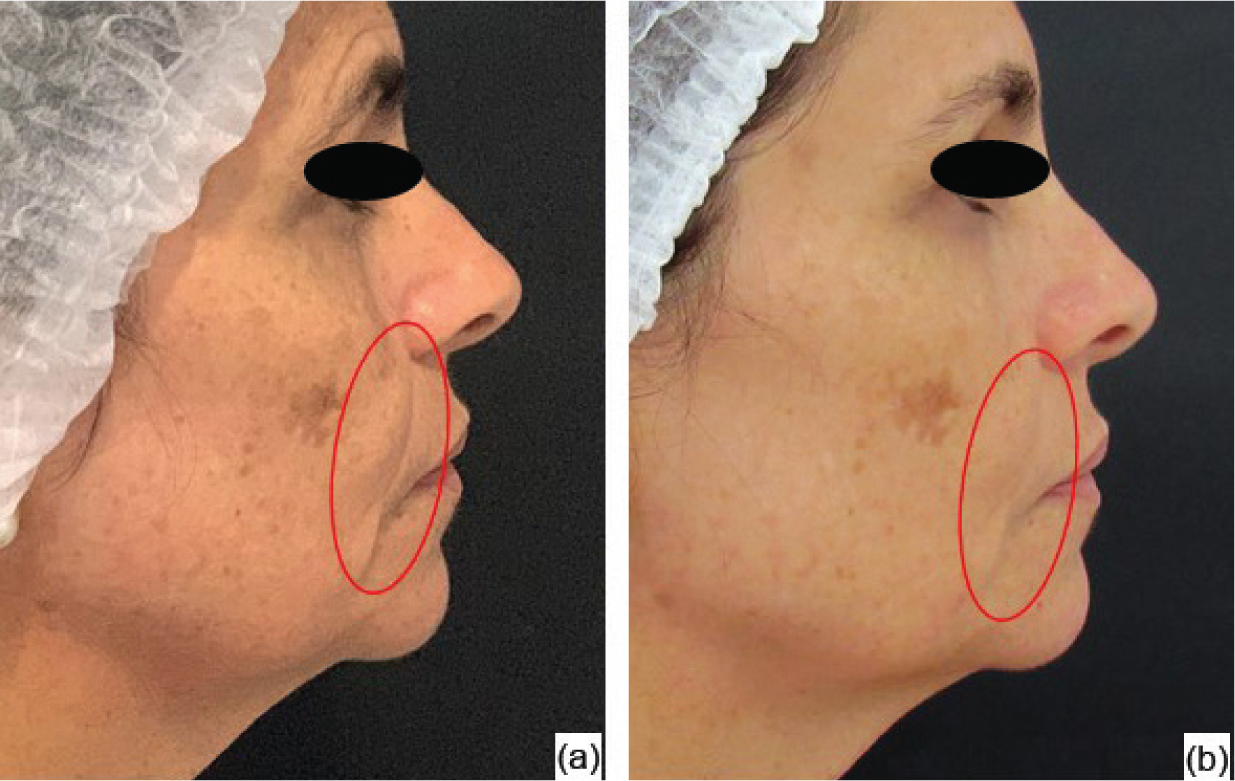 Figure 2. Group 1 volunteer: initial assessment (a) and evaluation after 45 days (b)
Figure 2. Group 1 volunteer: initial assessment (a) and evaluation after 45 days (b) 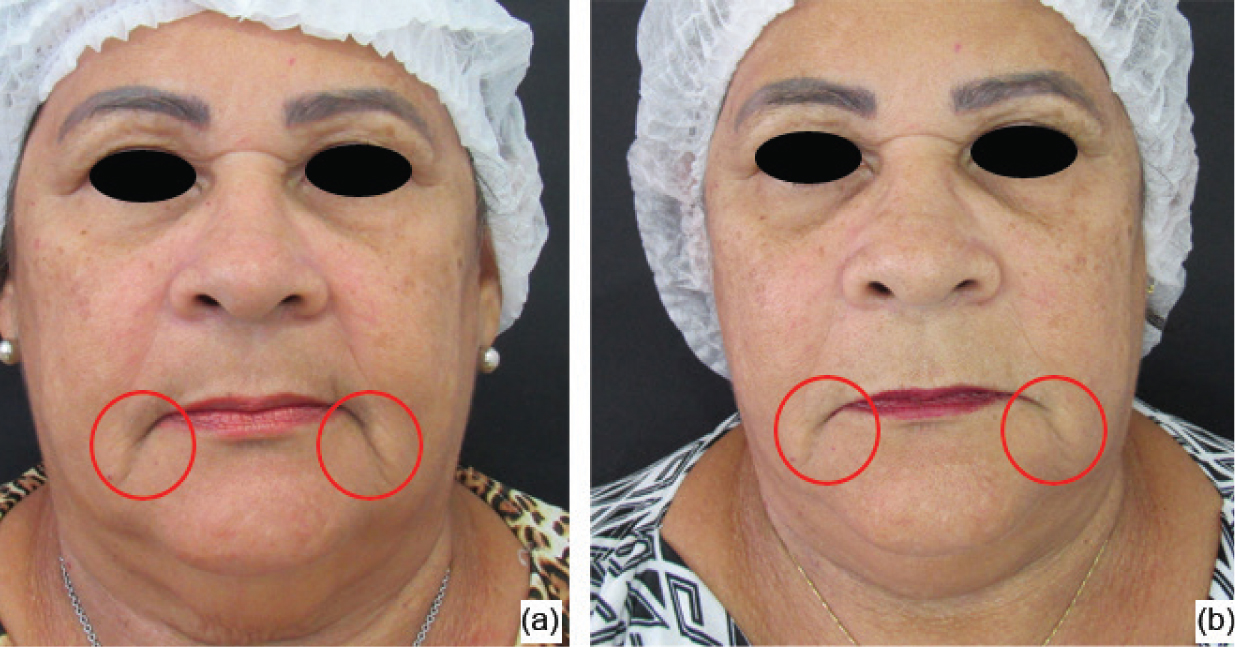 Figure 3. Group 1 volunteer: initial assessment (a) and evaluation after 90 days (b)
Figure 3. Group 1 volunteer: initial assessment (a) and evaluation after 90 days (b) 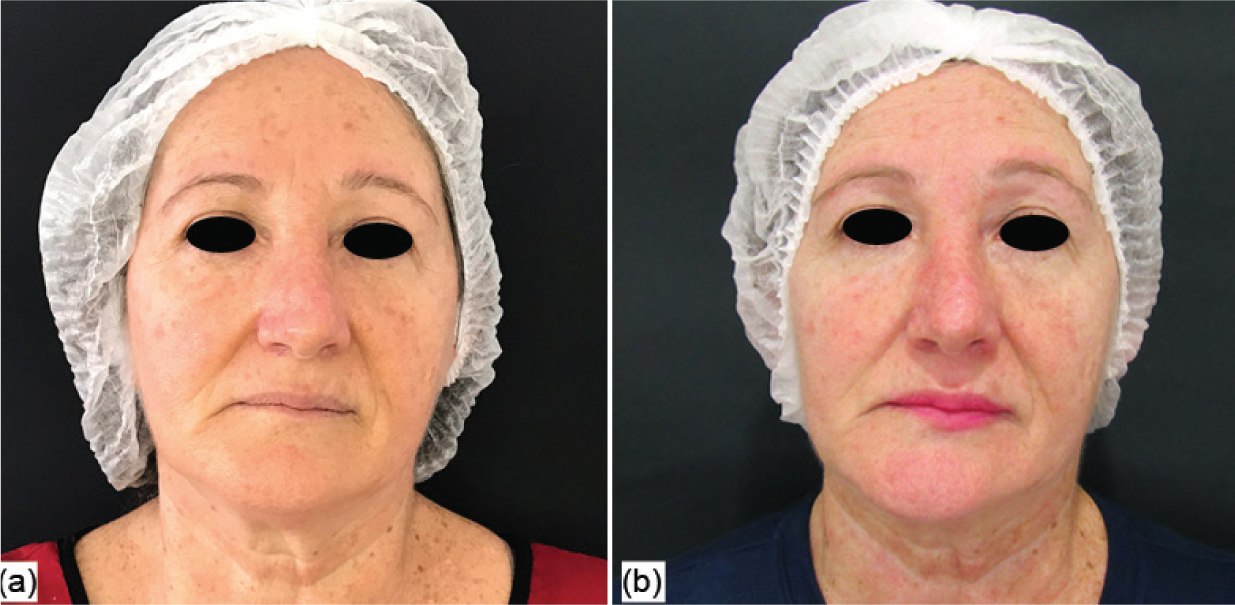 Figure 4. Group 2 volunteer: initial assessment (a) and evaluation after 45 days (b))
Figure 4. Group 2 volunteer: initial assessment (a) and evaluation after 45 days (b)) 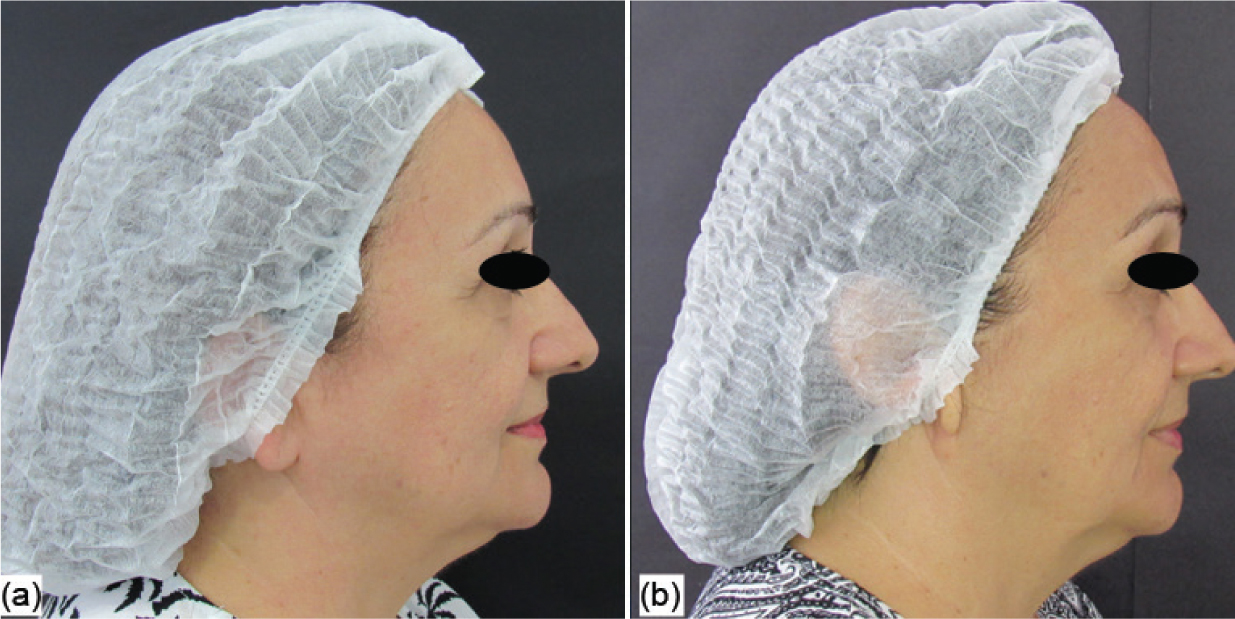 Figure 5. Group 2 volunteer: initial assessment (a) and evaluation after 90 days (b)
Figure 5. Group 2 volunteer: initial assessment (a) and evaluation after 90 days (b)
Table 2 highlights the results obtained through the adverse reaction and satisfaction questionnaire. Redness and burning were among the adverse reactions reported by the groups; however, these were of short duration. The presence of oedema on the face was present in most volunteers, with a maximum duration of 6 hours being reported. Both groups had a prolonged inflammatory response over a maximum period of 24 hours. It is noteworthy that volunteers from both groups complained of pain during the application, even with the use of the anaesthetic. There were no reports of other adverse reactions. Approximately 44% of G1 observed a difference in the areas treated at some point in the therapy, with that perception being more pronounced in the first two sessions. Similarly, although they were not identified in Figures 4 and 5, a large part of the control group also noticed a difference in the areas treated in the first two sessions.
Table 2. Results of the adverse reactions and satisfaction questionnaires
| Evaluation | Result (%) | ||
|---|---|---|---|
| Group 1 | Group 2 | ||
| Hyperaemia in the treated area | Yes | 33% | 33% |
| No | 67% | 67% | |
| Burning in the treated place | Yes | 17% | 17% |
| No | 83% | 83% | |
| Oedema on the face after applications | Yes | 25% | 17% |
| No | 25% | 33% | |
| Oedema lasted approximately 1 hour | 42% | 33% | |
| Oedema lasted approximately 2–6 hours | 8% | 17% | |
| Perception of difference in treated areas | Yes | 13% | 0% |
| No | 56% | 17% | |
| In the first two sessions | 18% | 66% | |
| In the first four sessions | 13% | 17% | |
For the topic ‘Skin texture improvement’ (Figure 6), in G1, most volunteers (67%) reported feeling the difference regarding texture, with it being classified as ‘much firmer’, while 33% did not feel any difference. G2 showed similar results.
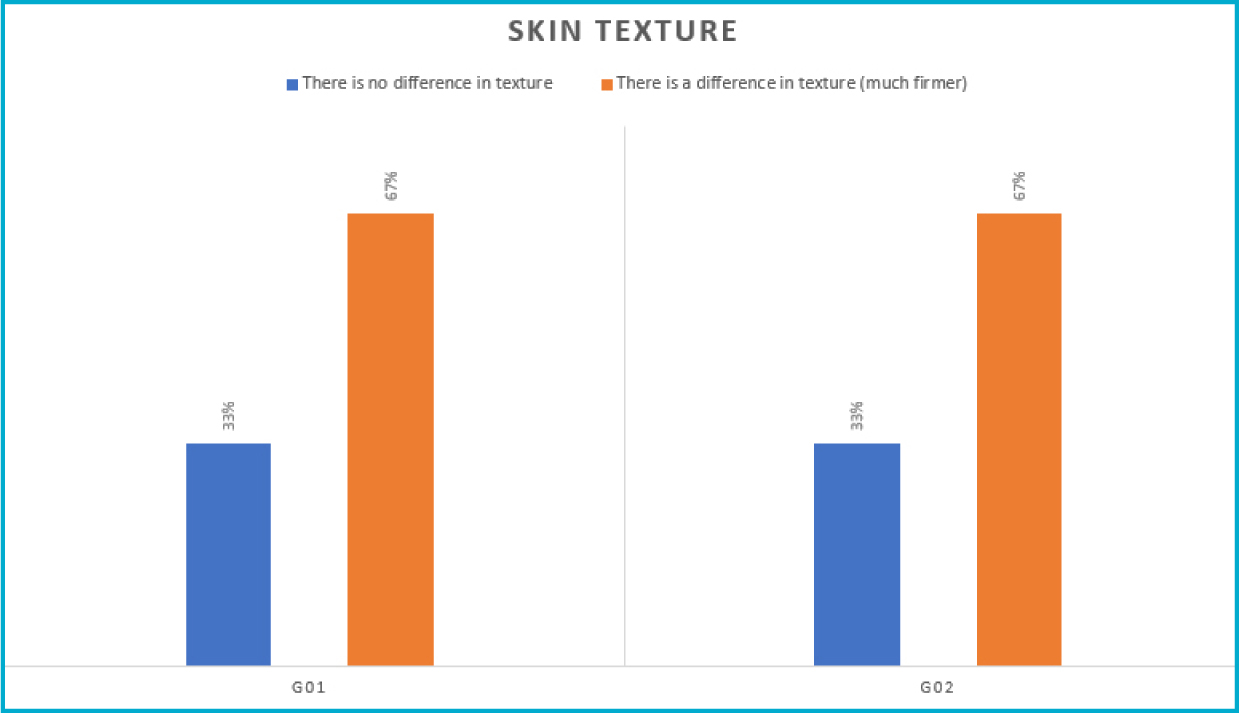 Figure 6. Skin texture improvement results
Figure 6. Skin texture improvement results
In the analysis of the questionnaire on treatment evaluation, in G1, 75% of the volunteers reported it as being an ‘excellent treatment’, while 25% reported it as being a ‘very good treatment’. In G2, 83% also reported it as being an ‘excellent treatment’ and 17% said it was a ‘very good treatment’. In both groups, all volunteers reported that they would do this aesthetic procedure again.
Discussion
Intradermotherapy uses injections of biocompatible and fully absorbable products into the superficial dermis to achieve skin rejuvenation, making it firmer, shinier and more hydrated (Lacarrubba et al, 2008). The signs of skin ageing are mainly related to extrinsic factors, such as exposure to solar radiation, and, as a consequence, the thickness of the epidermis, dermis and subcutaneous fat may decrease, causing expression lines, flaccidity and deep wrinkles to appear (Menoita et al, 2013). In the present study, there was a greater presence of static wrinkles and sagging, with signs of advanced photoageing (Glogau type III), and the wrinkles were classified as superficial for the treated group and deep for the control group.
The study by Savoia et al (2013) showed clinical and histological results from the use of intradermotherapy in facial rejuvenation. The treatment was carried out in 50 volunteers, using two formulations that contained hyaluronic acid and other substances. After 4 sessions at 15-day intervals, a significant clinical improvement was observed in regard to the brightness, texture and firmness of the skin. In the immunohistochemical analysis, there was an increase in the expression of type 1 collagen, suggesting that the formulation was able to interfere in the production of collagen in the injured skin.
In this study, the treated group showed improvement in the signs of facial ageing 45 days after the start of treatment, and this result improved and was better observed after 90 days. Amin et al's study (2006) used a different interval between sessions from that proposed by the authors' study; four sessions were performed at monthly intervals, using a multivitamin formulation added to hyaluronic acid. The photographic evaluations did not show any significant clinical differences; also, the histological analysis showed that the collagen fibres presented a smaller diameter after the treatment, concluding that the therapy with the product used did not present any significant benefit. However, the authors emphasise that the study did not make it clear which formulation was used.
The anti-ageing substance used in this study is composed of the transforming growth factor (nanofactor TGF-β3) and the epidermal growth factor (nanofactor EGF). These growth factors are characterised by being biologically active molecules, which act in the direct and external regulation of the cell cycle. At cell membrane level, they act by triggering a biochemical cascade with direct action on the cell nucleus, favouring genetic transcription. It is believed that its application would help to promote balance between the aged skin recovery processes, and, consequently, it would promote their interaction with the cells responsible for producing the extracellular matrix and the remodeling step, becoming beneficial in the rejuvenation process (Balbino et al, 2005; Vermolen et al, 2006; Eming et al, 2007; Mehta and Fitzpatrick, 2007).
For the treatment of photoaged skin, intradermotherapy can also be used with the application of platelet-rich plasma (PRP). For this technique, one uses a small amount of blood taken from the patient, which is then centrifuged at high speed, and the resulting platelet concentrate is injected intradermally to directly supply growth factors and cytokines to a target tissue, with a concentration 5-to 10-times higher. Thus, the growth factors will increase the proliferation of fibroblasts and the production of carboxylic peptide for collagen type 1 by dermal fibroblasts, improving the structural integrity of the skin. Another effect of PRP is inducing the expression of matrix metalloproteinases, which act by breaking and removing collagen fragments that interfere with the new formation of collagen in aged skin. However, the full safety of PRP as a component of intradermotherapy is yet to be proven and requires further studies (Konda and Thappa, 2013; Fabi and Sundaram, 2014).
Hyaluronic acid is considered a physiologic-pH polyanionic polymer, a characteristic of which is binding with water volumes corresponding to 1000-times its weight; thus, it helps the skin retain and maintain water, and is considered a natural free radical scavenger. In its natural state, this substance presents limited biomechanical properties as a skin filler. However, its biocompatibility and affinity for water molecules are excellent and, for this reason, hyaluronic acid is injected directly into the dermis to increase the water content of the skin and compensate for its endogenous lack (Ghersetich et al, 1994; Rieger, 1998).
Lacarrubba et al's study (2008) reported the application of multiple hyaluronic acid microinjections on the backs of the hands of 20 women, who had moderate physical signs of photoageing in that area. After four weekly sessions, the results evaluated by means of skin ultrasound were positive. The authors considered this therapy to be effective in the treatment of skin photoageing; however, the need for further studies on this association was highlighted.
Another compound used in this study was organic silicon, which is present in various tissues in the human body. Its nutritional deficiency leads to a reduction in collagen synthesis and in the formation of proteins and glycosaminoglycans in bones and cartilage. Thus, it is suggested that its use may stimulate the synthesis of elastin and collagen fibres, leading to a remodelling of the dermis structure (Carlisle, 1972; Vedamurthy, 2007).
The application of this substance was tested by Guillaume et al (1984). For this, intradermal injections of silanol salicylate (organic silicon) and saline solution were applied to the skin of women with moderate photoageing, and then subject to histological analyses. The treated group showed an increase in the number of elastic and collagen fibres, and the collagen texture became more homogeneous. Another study evaluated the application of 0.5% silanol salicylate using the intradermotherapy technique for the treatment of atrophic scars. After five application sessions, they observed scar-filling, improved skin texture and patient satisfaction (Herreros et al, 2009).
The literature states that, during the application of intradermotherapy in ideal conditions, there might be some exceptional complications, which would be related to particular characteristics or individual hypersensitivity. Thus, it is essential to assess the risks/benefits of the clinical use of this therapy to prevent the occurrence of unwanted reactions (Vega-López et al, 2016). The volunteers in this study observed the presence of hyperaemia, burning, oedema on the face and prolonged inflammatory response, although these reactions were short-lived; additionally, there were reports of painful sensation during the application, even with the use of the anaesthetic. One study indicated that, after the intradermotherapy sessions, the volunteers presented mild facial erythema and oedema secondary to the traumatic action of the needle and injected substances, with spontaneous resolution within 48 hours. Minimal discomfort during treatment has also been reported, but without post-treatment pain (El-Samahy et al, 2015).
The evaluation of the technique indicated that the effects were positive and there was an improvement in skin texture, felt by both groups, which reported that the skin was ‘much firmer’ when compared to the beginning of the study. Another relevant point was the classification of the therapy as an ‘excellent treatment’ and a ‘very good treatment’. Furthermore, all the volunteers reported that they would have this medical aesthetic procedure again. Thus, studies carried out on intradermotherapy are demonstrating its effectiveness as a non-invasive therapeutic procedure for facial rejuvenation, which is well tolerated by patients. This was demonstrated by another study, in which, after using intradermotherapy for six sessions at 15-day intervals, volunteers reported a 60% improvement in the overall appearance of the facial skin, which presented lighter and more uniform skin tone. They also reported that fine lines and wrinkles seemed to be minimised and the signs of ageing had significantly decreased (El-Samahy et al, 2015).
The data presented by the control group did not demonstrate significant clinical changes; however, a large part of the group reported being satisfied with the results, with this outcome being related to the influence of the placebo effect. According to Teixeira (2009), this is due to the stimulation of the technique, the expectations created in the treatment and the care and attention given to the participants. This placebo effect was also observed in a study that used the intradermotherapy technique to treat androgenetic alopecia in 90 volunteers (Sobhy et al, 2013), where two groups were treated with dutasteride (pure and in solution) and the third group was treated with saline. After nine treatment sessions, when performing the self-assessment analysis, all groups were satisfied, without significant differences. Additionally, most patients, including those who were not satisfied, requested more sessions, explaining the psychological aspect of therapy.
It is noteworthy that this study was subject to certain limitations, such as the impossibility of comparing the effects with other studies using dosages equal or very similar to those used in this protocol, as this parameter varies greatly in the literature and, in some cases, is not described in detail by the study methodology. Also, few quantitative assessment tools were used, with part of the results based on the subjectivity of the volunteers, who were influenced by the placebo, thus becoming a methodological flaw. Finally, there was a sample loss during the procedures, which made it impossible to distribute the volunteers equally in the proposed groups. Additional studies are suggested to clarify and explain their action mechanisms, using similar parameters and new treatment associations.
Conclusion
Intradermotherapy, using the conventional injection method, showed favourable results in the treatment of facial rejuvenation in women, with improvement in skin texture and reduction of wrinkles, when compared to the group that was treated only with saline, with the results even more evident 90 days after the initial application.
CPD reflective questions
- Can you change your practice to improve patient outcomes? How? If not, why?
- What do you need to do to implement change in your practice?
Key points
- Growth of non-surgical treatments is leading to the constant advancement of interest in non-invasive treatments
- The anti-ageing substance proved to be efficient in several points of the face, when compared with the placebo group
- Intradermotherapy using the conventional injection method showed favourable results in the treatment of facial rejuvenation in women.



